Synthesis and Characterization of Iridium Organometallic Complexes Covalently Grafted to Mesoporous Silicates and their Application on Removal of Heavy Metal Ions from Aqueous Solution
Moawia O Ahmed
DOI10.21767/2471-9838.100021
Moawia O Ahmed*
Department of Chemistry, Faculty of Science, Taibah University, Al Madinah Al Munawarah, Saudi Arabia
- *Corresponding Author:
- Dr Moawia Omer Elhag Ahmed
Associate Professor of Organometallic Chemistry, Faculty of Science, Chemistry Department, Taibah University, Al-Madinah Al-Munawarah, Kingdom of Saudi Arabia.
Tel: +966-565548322
E-mail:moawia99@yahoo.com, moahmed@ taibahu.edu.sa
Received date: March 14, 2017; Accepted date: March 31, 2017; Published date: April 03, 2017
Citation: Ahmed MO. Synthesis and Characterization of Iridium Organometallic Complexes Covalently Grafted to Mesoporous Silicates and their Application on Removal of Heavy Metal Ions from Aqueous Solution. Nano Res Appl. 2017, 3:1. doi:10.21767/2471-9838.100004
Abstract
In the present paper, a cyclometalated thiolated bridged iridium dimmers [(L)2IrS(CH2)3OH]2 where L is a bidentate cyclometalated ligand, 2,5-di(thiophen- 2-yl) pyridine (dtp) (1) and 2-benzo [4,5] thienyl pyridine (btp) (2) were covalently bonded into silicate framework by hydrolysis and condensation reaction. The new nanohybrid materials were fully characterized by Fourier trans-form infrared spectroscopy (FTIR), X-ray diffractometer (XRD), transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The results confirmed successful doping of the silicates with phosphorescent iridium organometallic complex. The TEM studies have suggested the nanoparticles nature. The prepared nanohybrids were found to be effective adsorbent for the removal of metal ions such as chromium (Cr2+), copper (Cu2+), cobalt (Co 2+), lead (Pb2+) and zinc (Zn2+) ions and from aqueous solutions.
Keywords
Heavy metals; Cyclometalated thiolated bridged dimer; Cyclometalated thiolated bridged dimmers; Nano-hybrid materials
Introduction
Wastes containing traces of toxic metal ions such as chromium, copper, cobalt lead and zinc etc., when discharged in the environment through chemical manufacturing, power generation, battery industry and welding etc., can cause serious environmental problem and pollution. Furthermore, contaminated waste can also cause a variety of diseases that would threaten human life as well as other animals [1-3].
A different number of methods are available for the removal of heavy metals from wastewater including, electrochemical treatment, chemical precipitation, ion-exchange, solvent extraction membrane technology, ultrafiltration, adsorption on activated carbon etc. However, most of these methods are ineffective, expensive and inapplicable to a wide range of pollutants [4-8].
In recent years, the synthesis of novel adsorbents for removal of toxic heavy metals from contaminated water has been a subject of interest for industrial research. There are several different types of adsorbents currently being used and proposed for adsorption of heavy metals ions such as clays, ion-exchange resins and activated carbon. However, these adsorbents have shown several disadvantages like poor mechanical stability, low thermal stability as well as low adsorption capacities and poor selectivity [9-11]. Recently, organic-inorganic hybrid materials have emerged as promising adsorbents for heavy metals overcoming the limitations of current adsorbents. This is due to their strong affinities, high adsorption capacities towards selected metal ions, in addition, organic–inorganic hybrid containing silica has high chemical stability and high heat resistance [12,13].
Nanostructure organic-inorganic hybrid materials which contain both organic compounds and inorganic oxides were developed and have been widely used in numerous fields including catalysis, optics and in the manufacture of photoelectric components and electrochemical biosensors [14-17]. For example, Franville et al. prepared europium(III)-containing hybrid materials based on dipicolinic acids by hydrolysis and condensation, europium complex was covalently bonded into silica framework. They have shown that, the europium (III) complexes in the silica hybrid matrix has good thermal stability and optical performance [18-20]. Li Junfu et al. also have reported a series of organically modified silicates doped with red-emitting europium complex. Jingxia, has reported a pH sensing mesoporous material containing covalently bonded Ru(II) complex in the silicate [21,22].
In the present paper, a new nano-hybrid adsorbents have been designed and synthesized namely, cyclometalated thiolated bridged dimmers [(L)2 IrS(CH2)3OH]2, where L is a bidentate cyclometalated ligand, 2,5-di(thiophen-2-yl) pyridine (dtp) (1) and 2-benzo [4,5-]thienylpyridine (btp) (2) were covalently bonded into silicate framework by hydrolysis and condensation reaction involving the silanol groups (Si-OH) and the hydroxyl groups of the cyclometalated thiolated bridged dimer (4). The resulted phosphorescent amorphous nano-hybrid materials 1 and 2 were fully characterized by Fourier-transform infrared (FTIR) spectra, transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images. Their application on removal of heavy metals such chromium, copper, cobalt, lead and zinc ions from aqueous solution were also investigated.
Materials and Methods
2-methoxyethanol, 3-(triethoxysilyl) propane-1-thiol and IrCl3.3H2O were obtained from Aldrich and used as received. Chloride-bridged dimers [Ir [(L)2 (μ-Cl)]2 and the thiolated bridged iridium dimers [(L)2 IrS(CH2)3OH]2, where L is a bidentate cyclometallated ligand, 2,5-di(thiophen-2-yl) pyridine(dtp) (1) and 2-benzo[4,5-]thienylpyridine (btp) (2) were prepared as described in literature Nonoyama [23] and Kotera et al. [24].
Method for synthesis of compound 1
The thiolated bridged iridium dimer [(dtp)2 IrS(CH2)3OH]2 (1 eqv) (4) were added to a solution of deionized water/ EtOH (25.0 mL, 50% v/v). The resulting suspension was sonicated for 30 min. The 3-(triethoxysilyl) propane-1-thiol (2.5 eqv) (6) was then dissolved in a deionized water/EtOH (25.0 mL, 50% v/v) solution and added to the thiolated bridged iridium dimer. The mixture was stirred for 24 h at 60°C. The resulting yellow precipitation was filtered, washed several times with distilled water and EtOH and dried overnight at about 105°C.
Nano-hybrid characterization
Infrared spectra were recorded in the range 400 cm-1 to 4000 cm-1 on a Shimadzu 8400S instrument using KBr pellets. The crystalline phases were identified using a powder X-ray diffractometer (XRD P-6000-ShimadzuX-ray diffractometer) using Cu Kα radiation. Transmission electron microscopy (TEM) was performed on a JEOL 1400 microscope. Agilent Inductively coupled plasma mass spectrometry (ICP-MS) Model 7500 Ce was used to measure the concentration of the metal ions in aqueous solution.
Results and Discussion
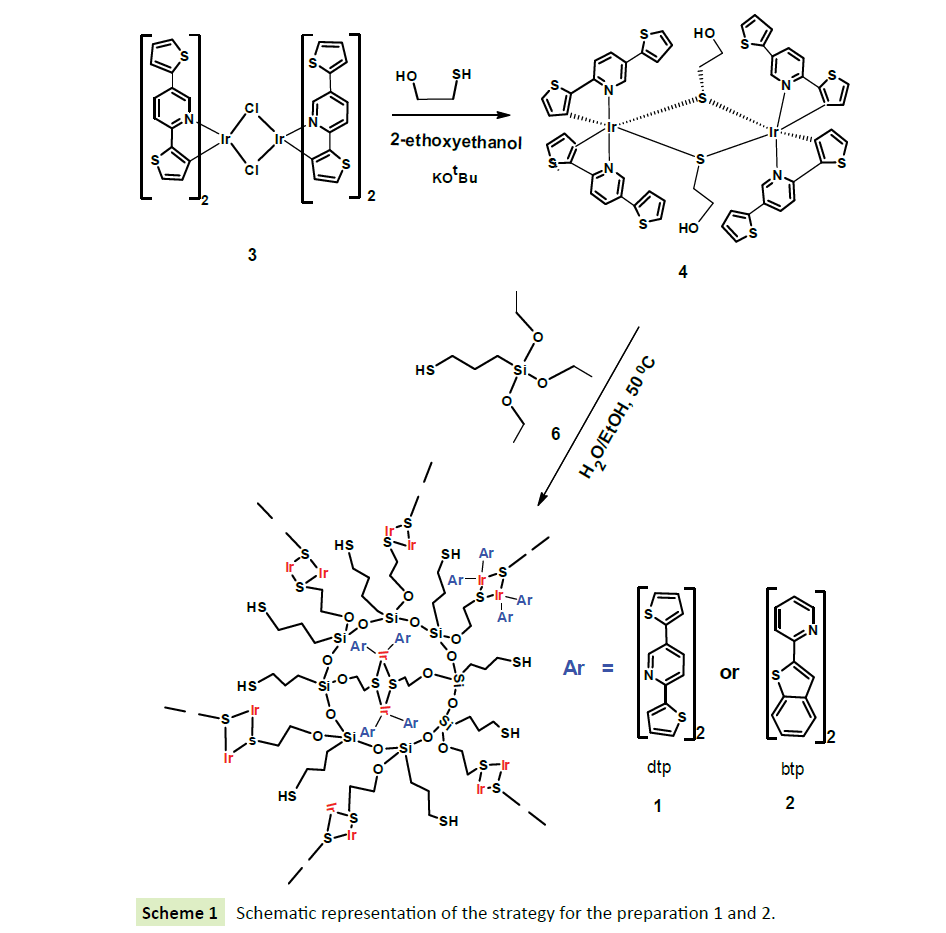
The complete synthesis tactic for the (EtO)3Si-SH@thiolate bridged iridium chloride dimer is shown in Scheme 1. The preparation involves two steps. First, the thiolated bridged iridium dimer (4) was prepared as described in literature by Kotera et al. [24]. In a typical reaction, addition of 2-mercaptoethanol and KOtBu in 2-methoxyethanol to a solution of [Ir [(btp2) (μ-Cl)]2 (3) in dichloromethane afforded the cyclometalated thiolated bridged dimer (4) as a yellow solid in a good yield. Second, the iridium (III)-doped hybrid material (1) was prepared by the reaction of hydroxyl groups on the cyclometalated thiolated bridged dimmer (4) with the hydrolyzed molecules of 3-(triethoxysilyl) propane-1-thiol (6) in ethanol/water mixture (50% v/v) at 50 °C for 24 h. Further hydrolysis of silicon ethoxide groups (Si-OEt) of compound 6 produces more silanol groups (Si-OH). Subsequent polycondensation reactions, involving the silanol groups (Si-OH) produced compound 1 as shown in Scheme 1.
For the synthesis of compound 2, the cyclometalated thiolated bridged dimmer [(btp)2 IrS(CH2)3OH]2 was mixed with 3-(triethoxysilyl) propane-1-thiol (6) under the similar conditions as that carried out for the preparation of (1). Both compounds 1 and 2 are insoluble in water and all organic solvents and have been fully characterized by Infra-red spectroscopy FTIR, TEM and SEM and XRD.
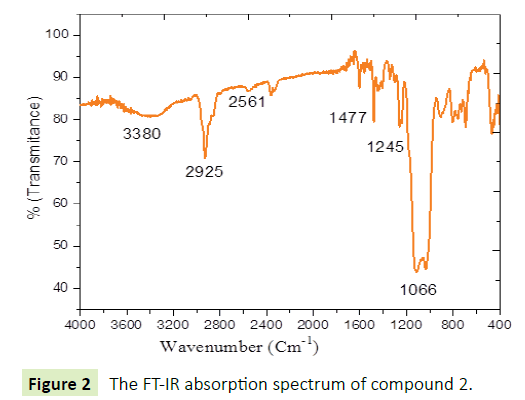
Figures 1 and 2 show the FT-IR absorption spectra of compounds 1 and 2, both figures display typical infrared spectra of hybrid materials, with both components, organic and inorganic phase. The inorganic components have strong absorption bands at 1068 cm-1, 1066 cm-1 for 1 and 2 respectively due to ν(Si-O-Si). The broad bands around 3500 cm-1 to 3000 cm-1 assigned to ν(Si- OH). SH stretching frequency appeared at 2561 cm-1 for both compounds 1 and 2. The organic components have absorption bands at 1244 cm-1 to 1477 cm-1 corresponding to aromatic rings stretching of pyridine and thiophene, bands at 2934 cm-1 and 29255 cm-1 due to aliphatic CH stretching modes for compounds I and 2 respectively [25-27].
Figure 3 shows the XRD spectra of compounds 1 and 2. XRD results indicated that both hybrid materials have no clear crystals phase peaks and exhibited typical silica amorphous structures with diffraction peaks at 2θ values around 21.5° [28].
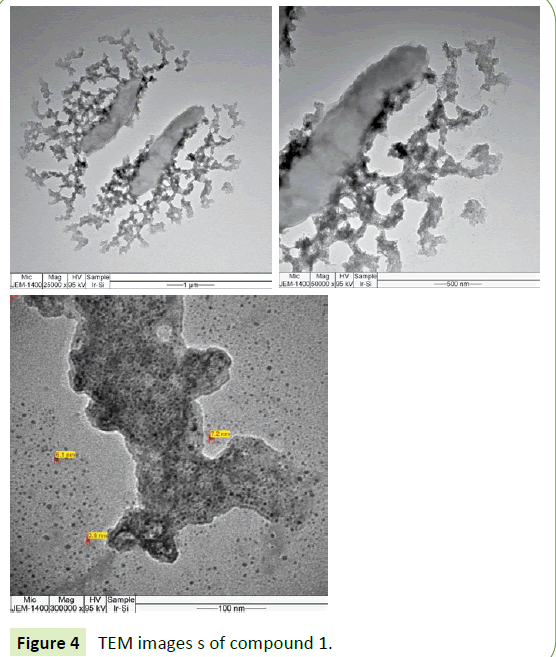
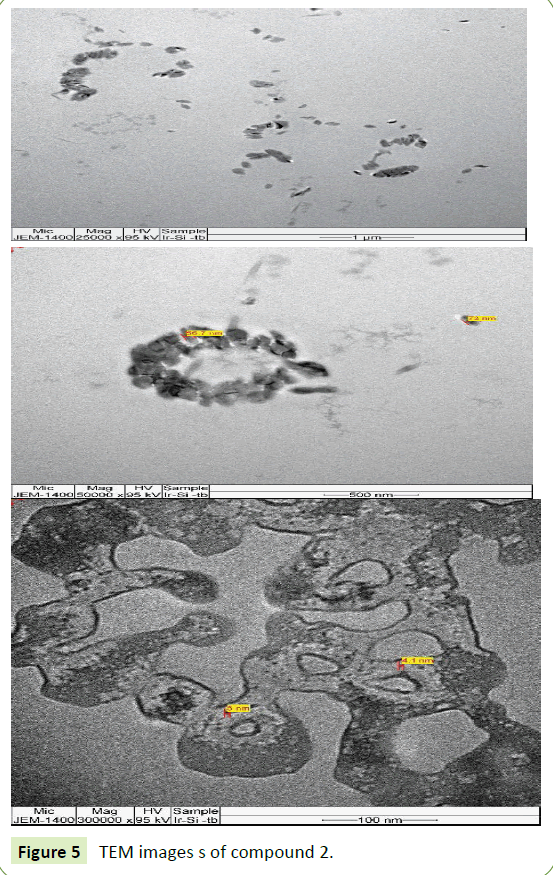
The TEM images of compounds 1 and 2 are shown in Figures 4 and 5 respectively. Both hybrid materials have particle sizes over a small range approximately from 3 nm to 7 nm in diameter.
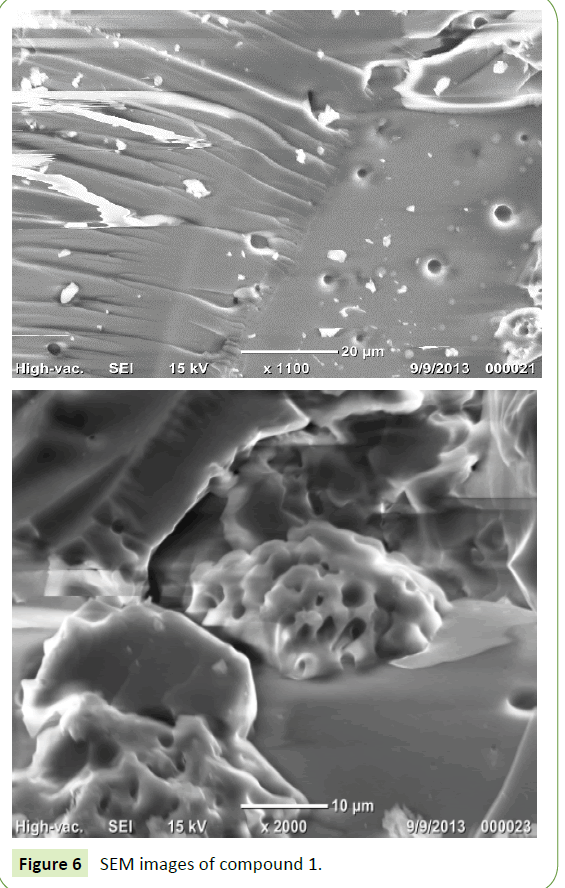
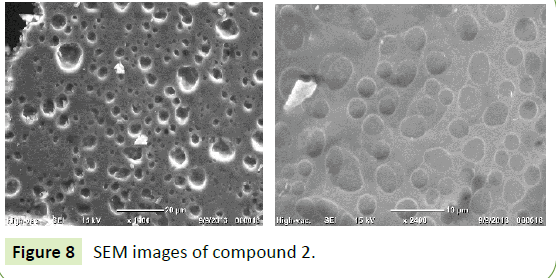
Figures 6-8 show SEM microphotographs obtained for both hybrid materials 1 and 2. The SEM results showed a different morphology, compound 1 has a rough surface and showed sponges of composite with pores, while compound 2 has a rough surface and dense body with pores. However, SEM images showed both nanocomposites had an amorphous structure which may be beneficial for adsorption of heavy metals. EDS spectra in Figures 7 and 9 confirmed the formation of compounds 1 and 2.
Adsorption capacity
The metal ion uptake capacity of Cr2+, Cu2+, Co2+and Pb2+ and Zn2+ metal ions by ICPMs was determined by mixing 50 mg of compounds 1 or 2 with buffered solutions of the divalent metal ions (1 ml of 1000 ppm) in 50 ml volumetric flask. Volume was made up to 50 ml with 2% nitric acid. With the help of stirrer, capacity by both adsorbents 1 and 2.
Conclusion
In this work, phosphorescent cyclometalated thiolated bridged iridium dimmers were immobilized in silicates by hydrolysis and condensation reactions. The new nanohybrid materials were fully characterized by IR, XRD and TEM. The results confirmed successful doping of the silicates with phosphorescent iridium organometallic complex. The powder XRD and TEM studies have suggested the nanoparticles nature. The prepared nanohybrids were found to be effective adsorbents for the removal of metal ions such as Cr2+, Cu2+ and Pb2+ and Zn2+ from aqueous solutions. samples were kept for 48 hours at speed of 50 rpm speed at room temperature. Samples were filtered with 0.45 mu syringe filter and analyzed by ICPMS Agilent 7500. Results obtained in ppm levels are given in Table 1 and Figure 10 below.
| Adsorbent | Adsorbent Capacity qt (mg/g )/(% ads) | ||||
| Cr2+ | Cu2+ | Co2+ | Pb2+ | Zn2+ | |
| 1 | 16.41 | 16.81 | 16.44 (82.20%) | 14.32 (71.60%) | 17.15 (85.75%) |
| -82.05% | -84.05% | ||||
| 2 | 16.35 | 16.89 | 16.58 | 14.54 | 17.14 |
| -81.75% | -84.45% | -82.90% | -72.70% | -85.70% | |
Table 1 Adsorption capacities (qt) and percent adsorption (% ads) of Cr2+, Cu2+, Co2+, Pb2+ and Zn2+ ions from aqueous solutions by 1 and 2.
The amount of each (II) ions adsorbed per unit weight of adsorbent (qt) and the percent adsorption (% ads) are calculated by using respectively the following equations [29].
(% ads) = (C0 – Ct)/ C0 x 100 and qt = [(C0 – Ct)] *V/W
Where C0 and Ct are the initial concentration and concentration at time contact of interaction adsorbate-adsorbent of each (II) ions. V is the volume of each metals solutions and W is the weight of Nano compounds.
The results show that Nano-hybrid materials 1 and 2 exhibit good potential for extraction of chromium, copper, cobalt, lead and zinc metal ions. This could be attributed to the change of morphology and increased in the surface area of the silica by the organometallic iridium doping. In addition, the presence of the immobilized thioyl group (SH) on the silica surface enhances the ion exchange process through acid-base interactions [30]. The results in Table 1 and Figure 10 show that zinc ions have the largest maximum adsorption capacity by both adsorbents 1 and 2.
Acknowledgment
The author is grateful to Dr Awad Asuhaimi and Mr. Manssor Sheik for the ICP-MS measurements.
Graphical Abstract
New phosphorescent amorphous nano-hybrid materials 1 and 2 were designed, synthesized and fully characterized by Fourier-transform infrared (FTIR) spectra, transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images. The new nanohybrids were found to be effective adsorbent for the removal of metal ions such as chromium (Cr2+), copper (Cu2+), cobalt (Co 2+), lead (Pb2+) and zinc (Zn2+) ions and from aqueous solutions.

References
- Bradl HB (2005) Heavy Metals in the Environment: Origin, Interaction and Remediation; Elsevier: Amsterdam, The Netherlands.
- Volesky B (1990) Biosorption and biosorbents of heavy metals. CRC Press, Boca Raton, Florida 3-5.
- Fergusson JE (1990) The Heavy Elements: Chemistry, environmental impact and health effects; Pergamon Press: Exeter, UK
- Esalah OJ, Weber ME, Vera JH (2000) Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium di-(n-octyl) phosphinate. Can J Chem Eng 78: 948-954.
- Emamjomeh MM, Sivakumar M (2009) Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J Environ Manag 90: 1663-1679.
- Yurloval L, Kryvoruchko A, Kornilovich B (2002) Removal of Ni (II) ions from wastewater by micellar-enhanced ultrafiltration. Desalination 144: 255-260.
- Matheickal JT, Yu Q (1999) Biosorption of lead (II) and copper (II) from aqueous solution by pretreated biomass of Australian marine algae. Bioresour Technol 69: 223-229.
- Benit Y, Ruiz ML (2002) Reverse osmosis applied to metal finishing wastewater. Desalination 142: 229-234.
- Gier S, Johns WD (2006) Heavy metal-adsorption on micas and clay minerals studied by X-ray photoelectron spectroscopy. Appl Clay Sci 16: 289-299.
- Nagarale RK, Gohil GS, Shahi VK (2006) Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci 119: 97-130.
- Uzun I, Güzel F (2000) Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk J Chem 24: 291-297.
- Zhou W, Mark JE, Unroe MR, Arnold FE (2001) Toughening of a high-temperature polymer by the sol-gel, in situ generation of a rubbery silica-siloxane phase. J Appl Polym Sci 79: 2326-2330.
- Hegde ND, Rao AV (2007) Physical properties of methyl tri-methoxy silane based elastic silica aerogels prepared by the two-stage sol-gel process. J Mater Sci 42: 6965-6971.
- Hoffmann F, Cornelius M, Morell J, Fröba M (2006) Silica based mesoporous organic-inorganic hybrid materials. Angew Chem Int Ed 45: 3216-3251.
- Sanchez C, Julián B, Belleville P, Popall M (2005) Applications of hybrid organic-inorganic nanocomposites J Mater Chem 15: 3559-3592.
- Judeinstein P, Sanchez C (1996) Hybrid organic-inorganic materials a land of multidisciplinarity. J Mater Chem 6: 511-525.
- Han YH, Taylor A, Mantle MD, Knowles KM (2007) Sol-gel-derived organic-inorganic hybrid materials. J non-cryst solids 353: 313-320.
- Franville AC, Zambon D, Mahiou R, Chou S, Troin Y, et al. (1998) Synthesis and optical features of an europium organic-inorganic silicate hybrid. J C J Alloys Compd 275: 831- 834.
- Franville AC, Zambon D, Mahiou R, Troin Y (2000) Luminescence behavior of sol−gel-derived hybrid materials resulting from covalent grafting of a chromophore unit to different organically modified alkoxysilanes. Chem Mater 12: 428-435.
- Franville AC, Mahiou R, Zambon D, Cousseins JC (2001) Molecular design of luminescent organic-inorganic hybrid materials activated by europium (III) ions Solid State Sci 3: 211.
- Jingxia W (2014) Mesoporous MCM-41 embeded with Ru (II)-based chemosensor: Preparation, characterization, and emission variation towards pH. J Luminescence 151: 41-46.
- Junfu L, Qiming G, Shurun L, Liping W, Lill W (2013) Study on a series of silicates modified with emitting europium complex molecules covalently bonded with silica matrix. Opt Mater 35: 674-680.
- Nonoyama M (1974) Benzo(h)quinolin-10-yl-N Iridium(III) complexes. Bull Chem Soc Jpn 47: 767.
- Kotera M, Suzuki T (2010) Syntheses and crystal structures of mononuclear and dinuclear(pentamethylcyclopentadienyl) iridium (III) complexes containing 2-mercaptobenzimidazole. Inorg Chim Acta 363: 3602.
- Wang Y, Liu HY, Jhang (1989) Acid-catalysed isomerization of indol-3-yl sulphides to indol-2-yl sulphides: Unexpected intermolecular nature of the rearrangement. J Chem Soc Chem Comm 1878.
- Ahmed YMZ, El-Sheikh SM (2009) Influence of the pH on the morphology of sol-gel-derived nanostructured. SiC J Am Ceram Soc 92: 2724-2730.
- Lei B, Li B, Zhang H, Zhang L, Li W (2007) Synthesis, characterization, and oxygen sensing properties of functionalized mesoporous SBA-15 and MCM-41 with a covalently linked Ruthenium(II) Complex. J Phys Chem 111: 11291-11301.
- Lenaerts P, Storms A, Mullens J, d’Haen, Gorller-Walrand JC (2005) Thin films of highly luminescent lanthanide complexes covalently linked to an organic-inorganic hybrid material via 2-substituted Imidazo(4,5-f)-1,10-phenanthroline groups. Chem Mater 17: 5194-5201.
- Najafi MR, Rafati R (2011) Chemically modified silica gel with thiol group as an adsorbent for retention of some toxic soft metal ions from water and industrial effluent. Chem Eng J 168: 426-432.
- Huheey JE (1975) Inorganic chemistry principles of structure and reactivity SI Units Ed., Harper and Row.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences