Role of Nanomaterials and their Applications as Photo-catalyst and Senors: A Review
Preeti Singh, Abdullah M. M and Saiqa Ikram
Preeti Singh1, Abdullah M. M2* and Saiqa Ikram1
Department of Chemistry, Faculty of Natural Sciences, Jamia Millia Islamia, New Delhi-110025, India
Promising Centre for Sensors and Electronic Devices (PCSED), Department of Physics, College of Science and Arts, Najran University, P.O. Box-1988, Najran–11001, Saudi Arabia
- *Corresponding Author:
- Abdullah M. M
Promising Centre for Sensors and Electronic Devices (PCSED), Department of Physics, College of Science and Arts, Najran University, P.O. Box-1988, Najran–11001, Saudi Arabia- E-mail: sikram@jmi.ac.in; abdullahphyzia@gmail.com
Received date: October 15, 2015, Accepted date: December 03, 2015, Published date: December 10, 2015
Citation: Abdullah MM. Role of Nanomaterials and their Applications as Photo-catalyst and Senors: A Review. Nano Res Appl. 2016, 2:1.
Abstract
Lack of environmental sustainability is a vital and growing problem due to the issues: such as climate change, pollution, and disturbances associated with biodiversity. A major cause of these environmental threats is pollutants in the atmosphere. Semiconducting metal oxide nanostructures play an important role in developing smart materials that are well efficient for sensing and simultaneously destroy harmful chemical contaminants from our environment. This review article highlights some recent advances of nano-science in the area of environmental hazardous contaminant detection by sensing followed by the remediation; focused on water remediation especially through photo-catalysis. In addition, the state-ofthe- art research activities involved in importance of metal oxide and their various applications are also discussed. The compilation consists of three main sections vis a vis (a) semiconducting metal oxides and applications, (b) Photocatalysis: fundamentals, processes and mechanism and (c) sensors: processes, types and their respective applications to monitor levels of environmentally important parameters. Also an inclusion of a section on future trends which discusses some of the new approaches being used to improve the selectivity and sensitivity of metal oxide semiconductors. This review concluded the perspectives and outlook on the future developments in the metal oxide nanostructure research area as well as summarizes a comprehensive compilation of the work done in order to address the challenges followed by prevailing achievements till date.
Keywords
Semiconducting metal-oxides; Photo-catalysis; Sensing
Introduction
Nanotechnology is an emerging field that covers a wide range of technologies which are presently under development at nanoscale. Behavior of materials at the nanoscale as compared to macroscale often found to be highly desirable properties which are created due to size confinement, the dominance of interfacial phenomena, and quantum effects. These new and unique properties of nanostructured materials, nanoparticles, and other related nanotechnologies lead to improved properties such as catalysts, tunable photoactivity, increased strength, and many other interesting characteristics [1]. It plays a major role in the development of innovative methods to produce new products, to substitute existing production equipment and to reformulate new materials and chemicals with improved performance resulting in less consumption of energy and materials and reduced harm to the environment as well as environmental remediation. Environmental applications of nanotechnology address the development of solutions to the existing environmental problems, preventive measures for future problems resulting from the interactions of energy and materials with the environment, and any possible risks that may be posed by nanotechnology itself.
Use of pesticides, herbicides, dyes, solvents, etc., rapidly in agriculture and large scale industrial development activities are causing much trouble and concern for the scientific communities and environmental regulatory authorities around the world. These organic pollutants adversely affect the environment and are a major source of aesthetic pollution, eutrophication and ecological disturbance in aquatic life due to their toxicity and persistence. To safeguard of our environment, it is very important to detoxify these hazardous organic pollutants. Among several proposed techniques for wastewater treatment: photocatalytic oxidation process provides a route for the detoxification of various toxic and hazardous pollutants as well as remedies water.
In the present scenario all the researchers and scientist are getting more and more attracted towards the metal oxide (MO) nanostructures due to their technology important applications in electronic as well as optoelectronic devices, sensors, medicines and renewable energy sources. Reducing the size of materials (metal oxide) to nano-level imparts properties which are different from the bulk or crystalline form and these nanoparticles demonstrate behavior like an isolated atoms and molecules [2]. Therefore, semi-conducting metal oxides (SCMO) are more potential due to their capability to generate charge carriers on stimulation with the required amount of energy and used for environmental remediation as well as electronics. Various methods of synthesis of SCMO are Chemical vapor deposition technique, hydrothermal method, Laser ablation technique, and electro-deposition method, etc. Out of these techniques, hydrothermal method is to friendliness use because of its costreducing and easy to handle as well as chemically reactive at low temperature.
For healthy environment, it is very important to maintain the ecological balance, in which controlling the environmental toxic pollutants is of great importance. MO semiconductors nanostructures are inevitable materials due to their novel characteristics and potential applications such as catalysis, ionexchange, molecular adsorption etc. Their outstanding properties are due to huge exposed active surface area, high stability, quantum confinement consequence, and high porosity as well as permeability (mesoporous in nature). This affects the various technological advancements mainly the particular advantages over traditional sensing methods, such as lower sensitivity, higher response time, and cost effective [3]. MOs are very strong decoloring agent used as photocatalytic degradation of organic pollutants. It plays an effective role in the treatment of waste water effluents. These promising applications of MO characterized as charge transport in electronic structure stimulates its light absorption properties and made it possible to be utilized as photocatalyst as well as sensor [4]. Specially, the SCMO nanostructures based sensors demonstrated better efficiency for the detection of various hazardous chemicals such as hydrogen, acetone, ammonium hydroxide, formaldehyde, and chloroform etc. It is because of the fact that: the low dimension of metal oxide nanostructures allows very sensitive transduction of the liquid/surface interactions into a change in the electrochemical properties [5].
Accordingly, it’s a credible contribution of researchers towards the healthier environment to organize a set-up for the photocatalytic and sensing activity for removal and detection of health hazardous compound form environment as well remediation of water by using SCMO. This review article presents the importance of nanotechnology mainly in terms of cleaning and monitoring of the environment. Here we discussed SCMO and their application in water remediation by using as photocatalyst and sensing in the form of chemical, gas and biosensor/s.
Photo-catalysis: fundamentals, processes and mechanism
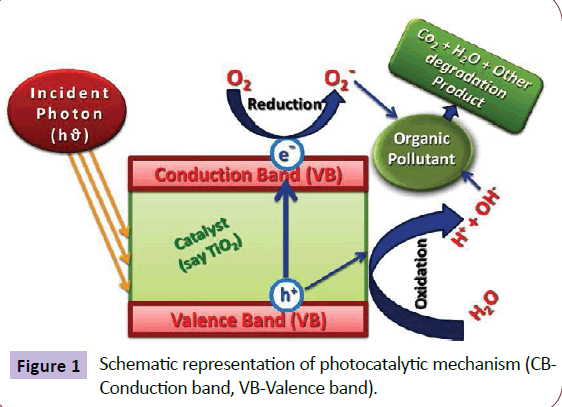
Catalysts are utilized for speeding up the chemical reaction and similarly, a photocatalyst employ the catalyst for speeding up chemical reactions in the presence of UV light. In this way, the absorption of light produces the electron–hole pairs that enable chemical transformations of the reaction participants and regenerate its chemical composition after each cycle of such interactions. There are two types of photocatalytic reactions, i.e., homogeneous photocatalysis and heterogeneous photocatalysis. Significant features of the photocatalytic systems are: proper band gap, morphology of the material, more exposed surface area, stability and its reusability. Photocatalysis is widely being practiced for the degradation and mineralization of hazardous organic compounds to CO2 and H2O and thus leads to the reduction of toxic metal ions into non-toxic states, deactivate and destruct all the water borne microorganisms, decomposes the air pollutants such as NO2, CO and NH3, degraded the waste plastics and green synthesis of industrially important chemicals. Thus, Photocatalysis refers to the oxidation and reduction reactions on the surfaces of photocatalyst material, mediated by the valence band (VB) such as holes(h+) and conduction band (CB) such as electrons (e-) generated by the absorption of UV-VIS light radiation. Such photo-generated pairs of h+ and e-induces the formation of aggressive species such as hydroxyl (OH-) or superoxide radicals from the moisture and atmospheric oxygen. These species are strong enough to oxidize and decompose organic materials or smelling gas and kill bacteria. Photocatalysis has been established as an efficient process for the mineralization of toxic organic compounds, hazardous inorganic materials and microbial disinfection as a result of the formation of the OH- ions, which acts as a strong oxidizing agent [6,7]. Figure 1 shows the schematic representation of photo catalytic mechanism. Briefly, the SCMO on irradiation with an appropriate wavelength (i.e., greater than or equal to band gap energy) lead to the excitation of an electron from the VB to the CB yielding an e-/h+ pair. When the reaction is conducted in the presence of water and oxygen, the electron in the conduction band is picked by oxygen giving rise to superoxide radical anion and in the oxidation site water is oxidized to give OH- radical. These are the two reactive species, which react with the organic pollutants leading to complete mineralization. The reactions of this e-/h+ pair with a variety of electron acceptors and donors, as well as e-/h+ recombination processes have been well-studied [8,9]. The formation of cation radicals of organic substrates, following electron transfer to excited semiconductors have been studied in several cases, both by product analysis [10] as well as by spectroscopic studies [11].
Extensive research has shown SCMO can photo-oxidise a wide range of organic substrates including alkanes, alkenes, aromatics, surfactants and pesticides [12-14]. Several MO such as TiO2, ZnO, MoO3, ZrO2, WO3, α-Fe2O3, SnO2, SrTiO3 and some chalcogenide metals (ZnS, CdS, CdSe, WS2, MoS2) can be used as photocatalysts which is an area of intensive research. However, in terms of thermodynamics, the valence band (VB) and conduction band (CB) in semiconductor which act as photocatalyst should be positioned in such a way that, the oxidation potential of the hydroxyl radicals and the reduction potential of superoxide radical lie well within the band gap. In other word, the redox potential of the VB hole must be sufficiently positive to generate OH- radicals and that of the CB electron must be sufficiently negative to generate superoxide radicals [15].
Fundamental Chemistry Involved in Photocatalyses
The standard reactions involved in the process of photocatalysis to degrade water contaminant or pollutant consist OH- radical to be the primary oxidant in the photocatalytic system and suggested the seven steps, all based on attack of OH-, lead to detoxification of harmful compound [16,17]. They follow:
(a) Photon energy greater than the band gap excites the catalyst and generates electrons and holes.
(b) Organic pollutants get adsorbed on the catalyst surface via lattice oxygen at the surface.
hν → e-+h+ (1)
h++H2O → H++OHÃÆââ¬Å¡Ãâ÷ (2)
h++OH-→ OHÃÆââ¬Å¡Ãâ÷ (3)
e-+O2 → O2- (4)
2e-+O2 + 2H+ → H2O2 (5)
e-+H2O2 → OHÃÆââ¬Å¡Ãâ÷+OH- (6)
Organic pollutant+•OH+O2 =CO2 +H2O+other degradation product form (7)
Sensor: processes, methods and mechanism
Metal oxides are being extensively studied due to their unique surface activities imparted by huge surface areas, which can make them ideal sensing elements as chemi-sensors. High surfaceto- volume ratios and high chemical and thermal stabilities of nanostructures under the operating conditions are responsible for their good sensing applications [18]. There are different types of sensors such as Chemical sensor, Bio sensor, gas sensor, optical sensor, magnetic sensor, temperature sensor, and humidity sensor etc. In this article we will discuss about three types: Chemical, Bio and gas sensors and their applications.
A sensor; in general is a technological device that detects or senses a signal, physical condition and chemical compounds. New and novel nano-materials have been playing a major role in the development of very sensitive, accurate, and reliable sensors. Nano-structured based devices have been found efficient in imaging and monitoring of nanomaterial, biological, chemical, and pathological samples. In the present scientific era, the development of sensors using nanostructured metal oxides, conducting oxides, semi-conducting oxides, polymers, and composites are the subject of study for detection and quantification of various hazardous gasses, chemicals and biochemicals [19].
Chemical Sensor
A chemical sensor is a device that transforms chemical information (composition, the presence of a particular element or ion, concentration, chemical activity, partial pressure...) into an analytically useful signal. The chemical information, mentioned above, may originate from a chemical reaction of analyte or from a physical property of the system investigated. They can have applications in different areas such as medicine, home safety, environmental pollution and many others. Chemical sensors usually contain two basic components connected in series: a chemical (molecular) recognition system (receptor) and a physicochemical transducer. Chemical sensing is quite commonly used in industry for process control and for monitoring, including monitoring for safety, plays important role in environmental protection, in racking of hazardous materials, tracking natural and man-made occurrences such as pollution, waterways infestation, migration of species, weather prediction and tracking etc.
Fabrication of chemical sensor is relatively very simple based on silicon processes or other thin or thick film technologies. The basic principle is that when an oxide is held at elevated temperatures, the surrounding gases react with the oxygen in the oxide causing changes in the resistivity of the material. The essential components are the high temperature, the oxide and the reaction in the oxide.
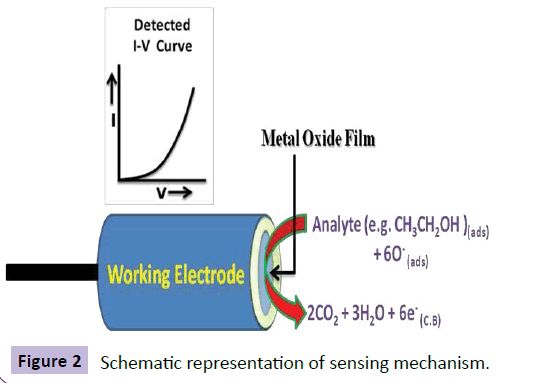
The principle of this mechanism (as shown in Figure 2) is based on redox reaction which takes place at the surface of MO nanostructures as shown in equation (8) and (9)
e- (MO nanostructures)+O2 → O2- (8)
e- (MO nanostructures)+O2- → 2O2- (9)
Thus, the sensitivity of analyte is accredited to the oxygen deficiency and increase in the oxygen adsorption on the surface of MO. The oxidation of analyte depends on the magnitude of adsorbed oxygen. If the adsorbed oxygen on the sensor surface be high, the oxidizing potential will be higher and the oxidation of chemicals (such as ethanol) will be faster. Negatively charged (adsorbed oxygen) oxidizes the chemicals (such as ethanol) to carbon dioxide and water and releases free electrons (6e-) to the conduction band of MO nanostructures as shown in equation below. These electrons increase the conductance of the film, and thus enhance the sensitivity.
Analyte (such as: CH3CH2OH) (ads)+6O- (ads) → 2CO2+3H2O+6e- (CB) (10)
Gas Sensor
Gas sensor measures the concentration of gas in its vicinity. Each gas has a unique breakdown voltage, i.e., the electric field at which it is ionized. Sensor identifies gases by measuring these voltages. The concentration of the gas can be determined by measuring the current discharge in the device. Various applications of gas sensor are such as process control industries, environmental monitoring, boiler control, fire detection, alcohol breath tests, detection of harmful gases in mines, home safety, grading of agro-products like coffee and spice. There are two operating parameters of gas sensor (1) operating temperature and (2) humidity. Also the disadvantages are: bulky, consume lots of power and require “risky” high voltage to operate. The detection principle of resistive sensors is based on change of the resistance of a thin film upon adsorption of the gas molecules on the surface of a semiconductor. The gas-solid interactions affect the resistance of the film because of the density of electronic species in the film.
Biosensor
Nanostructured metal oxides (NMOs) have recently become important as materials and have been found to exhibit interesting nano-morphological, functional biocompatible, non-toxic and catalytic properties, especially those of zinc, iron, cerium, tin, zirconium, titanium, metal and magnesium. These materials also exhibit enhanced electron-transfer kinetics and strong adsorption capability, providing suitable micro-environments for the immobilization of biomolecules and resulting in the enhanced electron transfer and improved biosensing characteristics as biosensors. To investigate the effect of optical and electrochemical properties of NMOs, suspended media, solid–liquid interfaces and nano-bio-interfaces are being conducted for biosensor applications.
A biosensor is an integrated miniaturized device that employs a biological element (antibody, enzyme, receptor protein, nucleic acid, whole cell or tissue section) as a sensing element coupled to a transducer for signal detection. A biosensor utilizes the selectivity of the biomolecule and the processing power of modern microelectronics and optoelectronics and is hence a powerful analytical tool with applications in medical diagnostics and other areas. Due to their specificity, portability, rapid response time and low cost, biosensors are projected to play a critical role in both clinical and non-clinical applications. The prevailing miniaturization tools allow packing of numerous microscopic electrodes along with transducers into a small footprint of a biochip device, leading to the design of high-density bioarrays. Several biosensors based on different detection for different analytes such as: hydrogen, ammonium and sodium ions as well as urea, creatynine, triglycerides, acetylcholine, butyrylcholine, pesticides and heavy metal ions were presented [20].Biosensors helps in detecting emerging contaminants like pharmaceuticals, personal care products (PPCPs), steroids, xenoestrogens, and other endocrine disrupting compounds (EDCs), algal toxins, giardia (and other pathogens), and a variety of miscellaneous chemicals such as caffeine, cholesterol [21], etc.
To fabricate an efficient biosensor, it is crucial to select an NMO that is suitable for immobilization of the desired biomolecule. The interface formed due to binding between an NMO and a biomolecule is known to affect the performance of a biosensor. The formation and properties of a nanobiointerface depend on the nature of the NMO; parameters like effective surface area, surface charge, energy, roughness and porosity, valence/ conductance states, functional groups, physical states and hygroscopic nature all affect the formation of a biointerface. In general, biomolecules bind with MO nanoparticles via physical adsorption or chemical binding. The physical adsorption of a biomolecule arising due to weak interactions (e.g., Van der Waals, electrostatic, physisorption) depends on the surface morphology, reaction medium and net surface charge. Short-range forces arising via charge, depletion and solvent interactions also play an important role in the preparation of a nanobiointerface. Covalent binding of a biomolecule to an NMO depends on the availability of functional groups, which can be prepared by appropriate engineering of chemical reactions [22,23].
Widely Used Semiconductor Metal Oxide as a Photo-catalyst and Sensor-material
Usually Zno and TiO2 are the most commonly used metal oxide for photocatalytic and sensing purposes. This might be due to its outstanding functional behavior of a potential electronic communicator with the designed devices. Therefore, a detailed discussion over these two compounds have been made and reported. And a little part has been discussed on three new new compounds Iron Oxide (Fe2O3), Gadolinium Oxide (Gd2O3) and Antimony Oxide (Sb2O4) on which a lot of work is required to be explored by researchers.
Zinc oxide (ZnO)
Zinc compounds have been used for at least 2,500 years in the production of useful alloys such as brass founding applications in making coins, kettles and decors. The most common application of zinc is in the galvanization industries, providing a protective coating. Zinc as Zinc oxide (ZnO), a common zinc compound formed when metallic zinc is exposed to the air and forms a protective coating that protects the rest of the metal. Zno is used in paints, some rubber products, cosmetics, pharmaceuticals, plastics, printing inks, soap and batteries, and many other things Oxides of zinc mainly exist in two different forms: ZnO and ZnO2. ZnO is the most stable and shows technologically high impact in devices as smart material. It exists in three different crystalline phases: wurtzite, zinc-blende and rock-salt. The ZnO is the most functional materials used for many applications such as biosensors, solar-cells [24], photocatalysis [25], light-emitting diodes [26], gas sensing [27], and field-emission. Various morphology of ZnO such as nanorods [28], nanowires [29], nanonails, nanopencils [30,31], nanobullets [32,33], nanotubes, nanocomb-like structure, nanoribbons [34-36], nanobelts [37], nanohelices [38], nanopins [39], nanoneedles [40], etc., are reported. It has been observed that the properties of the material vary with varying shapes and sizes.
A ZnO nanocapsules of average diameter ~ 80 to 130 nm based chemical sensor was used to detect the chloroform by I-V technique. The cell was fabricated by coating the ZnO nanocapsules on carbon working electrode and a wire of Pd as counter electrode. As prepared nanosensor exhibited the good sensitivity (0.478 μA.cm-2mM-1), low detection limit (6.67 μM) in the linear dynamic range (12 μM to 12 mM), and short response time (around 10 second) and also reported photocatalytic degradation of Acridine Orange (AO) synthesis by ZnO nanocapsule, found the effective degradation of AO up to 86.36% after 80 minutes of exposure to UV light [41].
ZnO nanorods (NR) of average diameter ~ 455 ± 20 nm based chemical sensor was used to detect the acetone by I-V technique. The sensor was fabricated by coating the ZnO NR on carbon working electrode and a wire of Pd as counter electrode. As prepared nanosensor exhibited the excellent sensitivity (~0.7595 μA.cm-2mM-1), with a lower detection limit (0.125 ± 0.02 mM) in the linear dynamic range (0.13 mM to 0.668 M) and fast response time around 10 second. Also, the photocatalytic degradation of AO by ZnO nanocapsules has been reported with an effective degradation of AO up to 93.8% (more than earlier reported in 2011) after 80 minutes of exposure to UV light whereas in the absence of photocatalyst no observable loss of dye was observed [42].
A ZnO nanoparticle were synthesized by hydrothermal treatment with starting materials (zinc chloride and urea) in the presence of ammonium hydroxide and utilize it to degradation of methylene blue (MB) and almost complete degradation (91.0%) takes place within 85 min of irradiation time. Nanoparticle synthesis of average diameter 80-130 nm based chemical sensor was used to detect the methanol by I-V technique. A cell was fabricated by coating the ZnO nanoparticles on carbon working electrode and a wire of Pd as counter electrode. As prepared nanosensor exhibited the good sensitivity (0.9554 μA.cm-2mM-1), with a low detection limit (0.11 mM) in the linear dynamic range (0.25 mM to 1.8 M) and fast response time around 10 second [43].
A ZnO-CeO2 nanoparticles were successfully synthesized by the simple and efficient low temperature process and employed as photocatalyst for the removal of environmental contaminants and degrade of acridine orange (AO) and methylene blue (MB) and reported 92.1% degradation for AO and 80.7% degradation for MB in 170 min of irradiation time. ZnO-CeO2 nanoparticles of average diameter ~ 50 ± 10 nm based chemical sensor was used to detect ethanol by I-V technique. A cell was fabricated by coating the ZnO-CeO2 nanoparticles on gold working electrode and a wire of Pd as counter electrode. As prepared nanosensor exhibited the good sensitivity (2.1949 μA.cm-2mM-1), low detection limit (0.6 ± 0.05 mM) in the linear dynamic range (1.7 mM to 1.7 M), and short response time around 10 second [44] again in the year 2011, 84.55% of AO and 48.65% of MB (in the presence of ZnOCeO2 nanostructures) are degraded after 170 min of irradiation time whereas in the absence of nanomaterial, no observable loss of dye is seen here also but degradation percentage has decreased as compared to earlier experiment. A ZnO-CeO2 nano-structures of average diameter ~ 50 to 200 nm based chemical sensor was used to detect ethanol by I-V technique. The sensor was fabricated by coating the ZnO-CeO2 nanoparticles on silver working electrode and a wire of Pd as counter electrode. As prepared nanosensor exhibited the good sensitivity (0.8331 μA.cm-2mM-1), low detection limit (0.16 mmol/l) in the linear dynamic range (0.17 mmol/l-1.7 mol/l), and short response time of around 10 seconds [45]. Hollow mesoporous ZnO nanoglobules based chemical sensor was used to detect pipredine by C-V technique. The cell was fabricated by coating the ZnO nanoglobules on carbon working electrode. As prepared nanosensor exhibited the good sensitivity (59.9 μAcm- 2mM-1), low detection limit (3.3 μM), and short response time of around 10 seconds [46]. Hollow mesoporous ZnO nanourchins based chemical sensor was used to detect the hydrazine by C-V technique. As prepared nanosensor exhibited the good sensitivity (42.1 μAcm-2mM-1), low detection limit (78.6 μM) [47]. A highly sensitive hydrazine chemical sensor based on a field-effect transistor (FET) has been fabricated by growing vertically-aligned ZnO nanorods directly on silver electrodes. The FET sensor demonstrated a high sensitivity of 59.175 μA cm-2 μM-1 and a low detection limit of ~ 3.86 nM [48]. The ZnO nanotube thin film was grown directly on fluorine doped tin oxide (FTO) and was used as working electrode for the fabrication of ethanolamine chemical sensor. The sensor demonstrated higher sensitivity of 37.4 × 10-4 μAcm-2mM-1 and the detection limit 19.5 μM [49]. ZnO nanopencils based chemical sensor was used to detect the liquid ammonia by I-V technique. As prepared nanosensor exhibited the good sensitivity (26.58 μA.cm-2mM-1), low detection limit (5 nM), and short response time of around 10 seconds [50].
Titanium dioxide (TiO2)
Titanium one of the lightest metal from the transition element series possess excellent mechanical and thermal properties due to the highest strength-to-density ratio in comparison of any other metallic element and is unusually resistant to corrosion. Titanium is often alloyed with aluminium, vanadium, copper (to harden), iron, manganese, molybdenum, and with other metals. It finds major applications in steel as an alloying element (ferro-titanium) and as a deoxidizer, to reduce carbon content. Applications for titanium mill products (sheet, plate, bar, wire, forgings, castings) can be found in industrial, aerospace, recreational, and emerging markets. Powdered titanium is used in pyrotechnics as a source of bright-burning particles. The recent applications are exploring towards the fabrication of nanostructures of titanium oxides as conducting materials.
Numerous studies have been reported in literature showing ZnO to be better photocatalyst for the detoxification of a large number of organic contaminants. Paulios and Tsachpinis [51] reported enhanced degradation of Reactive Black 5 in the presence of ZnO rather than other metal oxides; TiO2–UV100, TiO2 (Degussa P25), and TiO2/WO3. Paulios et al. [52] carried out the degradation of Auramine in batch reactor and found that the performance of ZnO was better than TiO2 (Degussa P25). Hoffman et al. [53] have reported an efficient generation of H2O2 by ZnO rather than by TiO2. Thus, degradation in the presence of ZnO may progress in three different ways viz., photocatalytic oxidation or oxidation with the simultaneous generation of H2O2 (photocatalytically), or a simultaneous process of both ways [54,55]. TiO2 is a relatively inexpensive catalyst easily activated by sunlight applied for photocatalytic oxidation of As (III) for treating arsenic contaminated groundwater [56].
A Mini-review has been presented by Kunal Mondal, and Ashutosh Sharma [57] on photocatalytic oxidation of pollutant dyes such as azo dyes, organic pollutant, PAH (Polycyclic Aromatic Hydrocarbons) which are known to be endocrine disruptors by using TiO2 and ZnO. U.G. Akpan [58], 2009 has also presented a review based on the effects of various operating parameters which affect the process: pH of the solution to be degraded, and the pH of the precursor solution (catalyst’s solution during preparation of catalyst), oxidizing agent, calcination temperature, dopant content, and catalyst loading on the photocatalytic degradation of textile dyes specifically using TiO2-based photocatalysts. As some reactive dyes are degraded at higher pH, while others at lower pH; hence in photocatalytic degradation of dyes in waste waters, the reaction should be undertaken at the proper pH. Oxidizing agents, calcination temperature and catalyst loadings are found to exert their individual influence on the photocatalytic degradation of any dye. They found out that sol–gel method is widely used in the production of TiO2-based photocatalysts because of the ability of sol-gel method to synthesize nanosized crystallized powder of the photocatalysts with high purity even at low temperature. The toxic effect of nano-TiO2 is found limited in aquatic environment and efforts are required towards the understanding of the particles contributing to toxic effects on bacteria, algae, invertebrates, and vertebrate species. Also, a possibility that other toxicants can be associated with TiO2 nanoparticles and therefore likely synergistic effects must be examined for additional toxicological concerns [59].
Morteza montazerozohori [60] (2012) presented the photocatalytic degradation of an organic dye emerald green using nano TiO2 and studied its kinetics also. The degradation of emerald green in the presence of effectual agents such as photocatalyst dose, pH and irradiation time, shows the complete degradation after 150, 50 and 30 min by use of optimal doses of nano TiO2 at various pH and Observe 3.3 × 10-2 min-1, 7.09 × 10-2 min-1 and 1.32 × 10-1 min-1 for pH of 7, 8 and 9, respectively. Finally, the L-H rate constant, kr, and adsorption–desorption constant, KA and dye degradation half times (t1/2) were evaluated at some basic pH.
TiO2 nanocrystals based chemical sensors was used to detect acetone [61]. TiO2 nanotubes with three different structures consisting of amorphous, anatase or anatase/rutile mixed phases based three different gas sensors were used for hydrogen sensing and it has been found that the structural properties of the TiO2 nanotubes make them a viable new gas sensing nanomaterial at room temperature [62]. A high sensitivity, chemical sensor based on titania coated optical fiber was used for ammonia sensing [63]. Sensing Performance of Precisely Ordered TiO2 Nanowire Gas Sensors Fabricated by Electron-Beam Lithography was used to detect the ethanol. Sensing response of the TiO2 nanowire sensor to ethanol was found higher at higher gas concentrations [64].
Iron oxide (Fe2O3)
Iron is found to be very reactive towards oxygen and water in normal air to give hydrated iron oxides, commonly known as rust. Unlike many other metals which form passivating oxide layers, iron oxides occupy more volume than the metal and thus flake off, exposing fresh surfaces for corrosion. Iron oxides and oxidehydroxides are widespread in nature, play a vital role in many geological and biological processes, and are extensively used by humans, e.g., as iron ores, pigments, catalysts, and in blood as hemoglobin.
Freely dispersed nano-, sonic-, bulk Fe2O3 were used for photocatalytic water oxidation under UV and visible irradiation and as a result all three materials (5.6 mg) evolved the O2 from water with 20 mM aqueous AgNO3 as sacrificial electron acceptor [65] .Enhanced photocatalytic activity of porous α-Fe2O3 films exhibited the excellent photocatalytic activity in hydrogen generation by water splitting under UV radiation, which is two orders better than that reported for α-Fe2O3 powders [66]. Fe2O3 photocatalyzes the oxidation of aniline to azobenzene under natural sunlight and UV irradiation in protic and aprotic solvents and exhibited sustainable catalytic activity [67].
A sensor made from the α-Fe2O3 decorated with multiwall carbon nanotubes was used for the detection of low concentration of ethanol vapours [68]. The gas sensing properties of the sensors based on the as-prepared Ag/Fe2O3 nanoparticles to different gas were investigated in detail [69].
Gadolinium oxide (Gd2O3)
The fundamental interest in rare-earth metals is enhanced by the unique interplay between the highly localized 4f states and the itinerant valence electrons, which in particular can result in different electronic and magnetic properties of surfaces and interfaces as compared to the rather well-known bulk properties. Gadolinium (III) oxide, commonly known as gadolinia is an inorganic compound with the formula Gd2O3. It is one of the most commonly available forms of the gadolinium as one of the rare earth elements, derivatives of which are potential contrast agents for magnetic resonance imaging (MRI).
Visible-light activities of Gd2O3 /BiVO4 composite photocatalysts exhibited the enhanced photocatalytic activities than the pure BiVO4 for degradation of methyl orange under visible-light irradiation [70]. As grown and annealed Gd2O3 were used to detect the ethanol chemical by using the I-V technique. The sensor based on as-grown Gd2O3 nanorods demonstrated the high-sensitivity of 0.6940 μAcm-2mM-1 with low detection limit (0.02 mM) and correlation coefficient (R=0.7345) in short response time [71] whereas, the sensor based on annealed Gd2O3 nanostructures demonstrated the sensitivity of 0.266 μAcm-2mM-1 with a low detection limit (52.2 mM) and correlation coefficient (R = 0.618) in short response time [72].
Antimony oxide (Sb2O4)
Antimony oxide is an inorganic compound often classified according to its oxidation state Sb(III) and Sb(V). Also forms a mixed-valence oxide compound, antimony tetroxide (Sb2O4), which features both Sb(III) and Sb(V). Application of antimony is consumed in flame retardants, making of bullets and bullet tracers, paint and glass art crafts and as a pacifier in enamel. As an alloy, it is used for the production of batteries, plain bearings and solders, as a stabilizer and a catalyst for the production of poly ethylene terephthalate. Antimony and its compounds should be handled precariously as inhalation of antimony trioxide (and similar poorly soluble Sb (III) dust particles such as antimony dust) is considered harmful and suspected of causing cancer and corrosive to skin.
The photo-catalytic activity of the synthesized α-Sb2O4 was evaluated by degradation of acridine orange (AO) which degraded almost 52.1% in 170.0 minutes. The analytical performances of the fabricated chloroform sensor demonstrated good sensitivity (1.154 μAcm-2 mM-1), lower-detection limit (~10.0 μM), longlinear dynamic range (12.0 μM to 1.2 mM) with good linearity (R=0.8457) in short response time (10.0 Sec) [73].
Very few reports have been published based on rare earth metal such as a dynamic electrochemical sensor fabricated by using PVC for sensing europium (III) [74] developed sensor is useful in analytical as well as biological samples such as urine and it can also work as an electrode indicator. Researchers have also reported on detection and removal of heavy metal such as mercury from waste water [75] fabricated Uranyl Selective Polymeric Membrane Sensor based on p-tert-butylbiscalix arene for determination of Thorium [76].
It has been observed that the sensitivity of electrochemical sensors depends upon many factors such as electrodes, shapes and sizes of nanostructures, and target analytes etc. High surface area, excellent absorbing and adsorbing behavior, bio-friendly nature and high electron communication characteristics of nanostructures may be the reason behind the high sensitivity of this sensing system.
Conclusion
Researchers have performed very extensively, promising and well defined work in the field of photocatalyses as well sensors. The application of semiconductor nanoparticles as photocatalysts is still limited by the fact that they respond only to UV-excitation thus still lot scope is left to work in above require field due to the some following grounds such as synthesis of UV-Visible light induced nano-photocatalyst with enhanced activities should be in a controlled manner as well as its production should occur at large scale to meet the requirements.
Thus, a continuous effort is required to extend the response of metal oxides to the visible light are expected to draw the attention of future research. Though some research groups have successfully fabricated the sensors, but the selectivity is still quite low. The sensing issues of extremely high sensitivity, selectivity and stability should be resolved.
Incorporation of these nanostructures citing their photocatalytic and sensing activities can bring inevitable advantages inherent to the nanoscale, namely, an ability to transduce information at molecular resolutions, enhance low signal levels, quicken response times, and continuously monitor environmental in consequence to the health changes in the form of sensors. Exploiting nanostructured sensors in environmental processes witnesses a continuing need to bridge the divide between the engineering advancements in sensing components and configurations, and the emerging needs. Hence, in conclusion, knowledge of the different synthetic procedures employed for the preparation of photocatalytic materials should be considered necessarily. The search for new photocatalysts having desired properties to induce the oxidation reaction of organic substrates or the pollutants under visible light irradiation should be encouraged.
References
- Mansoori GA, Rohani BT, Ahmadpour A, Eshaghi Z (2008) Environmental Application of Nanotechnology. Annual Review of Nano Research 2:1-73.
- Lin YM, Cronin SB, Rabin O, Ying JY, Dresselhaus MS (2001)Transport Properties of Bi1-xSbx Alloy Nanowires Synthesized by Pressure Injection. Applied Physics Letter 79:677-679.
- Khan MM, Adil SyedF, Al-Mayouf Abd (2015) Metal oxides as photocatalysts. Journal of Saudi Chemical Society.
- Tan OK, Cao W, Zhu W, Chai JW, Pan JS (2003) Ethanol sensors based on nanosized Fe2O3 with SnO2, ZrO2, TiO2 solid solutions. Sensors Actuators B:Chemistry 93:396-401.
- Rahman MM, Gruner G, Al-Ghamdi MS, Daous MA, Khan SB, et al. (2013) Chemo-sensors development based on low-dimensional codoped Mn2O3-ZnO nanoparticles using flat-silver electrodes. Chem Cent J 7: 60.
- Sherine OO, Gerald JM (2004) Nanostructured Materials for Environmental Remediation of Organic Contaminants in Water. Journal of Environmental Science and Health, Part A—Toxic/Hazardous Substances & Environmental Engineering 39:2549–2582.
- Khin MM, Nair AS, Babu VJ (2012) A review on nanomaterials for environmental remediation. Energy & Environmental Science. Science for Environment Policy.
- Grover R, Cessna AJ (Eds.) (1991) Environmental Chemistry of Herbicides Vol II, CRC Press in Boca Raton, Florida.
- Umar A, Rahman MM, Kim SH, Hahn YB (2008) Zinc oxide nanonail based chemical sensor for hydrazine detection. ChemCommun (Camb): 166-168.
- Fox MA (1991)Photoinduced electron transfer in arranged media. Topic in current chemistry159: 67-101.
- Rothenberger G, Moser J, Gratzel M, Serpone N, Sharma DK (1985) Charge Carrier Trapping and Recombination Dynamics in Small Semiconductor Particles. Journal of American Chemical Society 107:8054-8059.
- Fox MA, Chen CC (1981) Mechanistic features of the semiconductor photocatalyzed olefin-to-carbonyl oxidative cleavage. Journal of American Chemical Society 103:6757-6759.
- Fox MA, Lindig B, Chen CC (1982) Transients generated upon photolysis of colloidal titanium dioxide in acetonitrile containing organic redox couples. Journal of American Chemical Society 104:5828-5829.
- Shigwedha N, Hua ZZ, Chen J (2007) A new photon kinetic-measurement based on the kinetics of electron-hole pairs in photodegradation of textile wastewater using the UV-H2O2FS-TiO2 process. J Environ Sci (China) 19: 367-373.
- RW Mathews in E Pelizzetti and M Schavello (eds.) (1991) Photochemical Conversion and Storage of Solar Energy. Kluwer, Dordrecht 427-449.
- David F Ollis, Al-Ekabi H (1993)Photocatalytic Purification and Treatment of Water and Air: Proceedings of the 1st International Conference on TiO2 Photocatalytic Purification an (Trace Metals in the Environment).
- Asim N, Badeiei M, Ghoreishi BK, Ludin NA, Reza M, et al. (2012)New developments in photocatalysts modification: case study of WO3, proceedings in Advances in Fluid Mechanics and Heat & Mass Transfer. 10th WSEAS International Conference on Heat Transfer, Thermal Engineering and Environment (HTE '12) ISBN: 978-1-61804-114-2:110-116.
- Hoffmann MR, Martin ST, Choi W, Bahnemannt DW (1995) Environmental Applications of Semiconductor Photocatalysis. Chemical Reviews 95:69-96.
- Mohamed RM, McKinney DL, Sigmund WM (2012) Enhanced nanocatalyst. Materials Science and Engineering 73:1-13.
- Huang MH, Mao S, Feick H, Yan H, Wu Y, et al. (2001) Room-temperature ultraviolet nanowire nanolasers. Science 292: 1897-1899.
- Ozaki Y, Suzuki S, Morimitsu M, Matsunaga M (2000) Enhanced long-term stability of SnO2-based CO gas sensors modified by sulfuric acid treatment. Sensors Actuators B: Chemical 62: 197-220.
- Torbicz W, Pijanowska DG(2004) Semiconductor chemical and biochemical sensors, Progress in Electromagnetic Research Symposium. Pisa, Italy 31:847-850
- Gautam P, Suniti S, PrachiKumari A, Deepa M, et al. (2012) A review on recent advances in biosensors for detection of water contamination. International journal of environmental sciences 2:1565-1574.
- Solanki P R, Kaushik A, Agrawal VV, Malhotra BD (2011) Nanostructured metal oxide-based biosensors. NPG Asia Materials 3: 17-24.
- Bolink HJ, Coronado E, SessoloJM (2009) Efficient polymer light-emitting diode using air-stable metal oxides as electrodes. Advance Materials 21:79-82.
- Emeline AV, Kataeva GV, Panasuk AV, Ryabchuk VK, Sheremetyeva NV,et al. (2005) Effect of Surface Photoreactions on the Photocoloration of a Wide Band Gap Metal Oxide:? Probing Whether Surface Reactions are Photocatalytic. Journal of physical chemistry B 109:5175-5185.
- Chu D, Masuda Y, Ohji T, Kato K (2010) Formation and photocatalytic application of ZnO nanotubes using aqueous solution. Langmuir 26: 2811-2815.
- Lupan O, Pauporte T, Bahers T Le, Viana B, Ciofini I (2011) Wavelength-Emission Tuning of ZnO Nanowire-Based Light-Emitting Diodes by Cu Doping: Experimental and Computational Insights. Advance functional materials 21:3564-3572.
- Rout CS, Krishna SH, Vivekchand SRC, Govindaraj A, Rao CNR (2006) Hydrogen and ethanol sensors based on ZnOnanorods, nanowires and nanotubes. Chemical Physics Letters 418:586-590.
- Zeng H, Xu X, Bando Y, Gautam UK, Zhai T, etal.(2009) Template Deformation-Tailored ZnONanorod/Nanowire Arrays: Full Growth Control and Optimization of Field-Emission. Advance Functional Material 19:3165-3172.
- Vayssieres L (2003) Growth of Arrayed Nanorods and Nanowires of ZnO from Aqueous Solutions. Advance Materials 15:464-466.
- Shen GZ, Bando Y, Liu BD, Golberg D, Lee CJ (2006) Characterization and field emission properties of vertically aligned Znonanonails and nanaopencils fabricated by modified thermal evaporation process. Advance Functional Materials 16:410-416.
- Gautam UK, Panchakarla LS, Dierre B, Fang XS, Bando Y et al. (2009) Solvothermal Synthesis, Cathodoluminescence, and Field-Emission Properties of Pure and N-Doped ZnONanobullets. Advance Functional Materials19:131-140.
- WeiA, Sun XW, Xu CX, Dong ZL, Yu MB,et al. (2006) Stable field emission from hydrothermally grown ZnO nanotubes. Applied Physics Letters 88:102-213 .
- Wang N, Cao X, Guo L (2008) Facile One-Pot Solution Phase Synthesis of SnO2 Nanotubes. Journal of Physical Chemistry C112:12616-12622.
- Tseng YK, Huang CJ, Cheng HM, Lin IN, Liu KS et al. (2003) Characterization and Field-Emission Properties of Needle-like Zinc Oxide Nanowires Grown Vertically on Conductive Zinc Oxide Films. Advance Functional Materials 13:811-814.
- Wang W, Zeng B, Yang J, Poudel B, Huang J,et al. (2006) Aligned UltralongZnONanobelts and Their Enhanced Field Emission. Advanced Materials18:3275-3278.
- Gao PX, Ding Y, Mai W, Hughes WL, Lao C, et al. (2005) Conversion of zinc oxide nanobelts into superlattice-structured nanohelices. Science 309: 1700-1704.
- Xu CX, Sun XW (2003) Field emission from zinc oxide nanopins. Applied Physics Letters 83:3806-3808.
- Zhu YW, Zhang HZ, Sun XC, Feng SQ, Xu J,et al. (2003) Efficient field emission from ZnOnanoneedle arrays. Applied Physics Letters 83:144-146.
- Faisal M, Khan SB, Rahman MM, Khan A, Muneer M,et al. (2011) Synthesis, characterizations, photocatalytic and sensing studies of ZnOnanocapsules. Applied Surface Science 258:672-677.
- Faisal M, Khan SB, Rahman MM, Jamal A, Abdullah MM (2012) Fabrication of ZnO nanoparticles based sensitive methanol sensor and efficient photocatalyst. Applied Surface Science 258:7515-7522.
- Faisal M, Khan SB, Rahman MM, Jamal A, Asiri AM, Abdullah MM (2011) Smart chemical sensor and active photo-catalyst for environmental pollutants. Chemical Engineering Journal 173:178-184.
- Faisal M, Khan SB, Rahman MM, Jamal A, Akhtar K,et al. (2011) Role of ZnO-CeO2 Nanostructures as a Photo-catalyst and Chemi-sensor. Journal of Material Science and Technology 27:594-600.
- Ameen S, Shaheer AM, Seo HK, Shin HS (2015) An electrochemical sensing platform based on hollow mesoporousZnOnanoglobules modified glassy carbon electrode: Selective detection of piperidine chemical. Chemical Engineering Journal 270: 564-571.
- Ahmad U, Akhtar MS, Al-Hajry AS, Al-Assiri M, Dar GN,et al. (2015) Enhanced photocatalytic degradation of harmful dye and phenyl hydrazine chemical sensing using ZnOnanourchins. Chemical Engineering Journal 262:588-596.
- Ahmad R, Tripathy N, Jung DU, Hahn YB (2014) Highly sensitive hydrazine chemical sensor based on ZnOnanorods field-effect transistor. ChemCommun (Camb) 50: 1890-1893.
- Amin S, Akhtar MS, Shin HS (2013) Rapid photocatalytic degradation of crystal violet dye over ZnO flower nanomaterials. Materials Letters 106:254-258.
- Dar GN, Umar A, Zaidi SA, Baskoutas S, Hwang SW, et al. (2012) Ultra-high sensitive ammonia chemical sensor based on ZnOnanopencils. Talanta 89: 155-161.
- Poulios I,Tsachpinis I (1999) Photodegradation of the textile dye reactive black 5 in the presence of semiconducting oxides. Journal of chemical technology and biotechnology 74:349-357.
- Poulios I, Avranas A, Rekliti E, Zouboulis A (2000) Photocatalytic oxidation of Auramine-O in the presence of semiconducting oxides. Journal of chemical technology and biotechnology 75:205-212.
- Hoffman MR, Martin ST, Choi WY, Bahnemann DW (1995) Environmental Applications of Semiconductor Photocatalysis. Chemical Reviews 95:69-96.
- Hoffman AJ, Carraway ER, Hoffmann MR (1994) Photocatalytic Production of Hydrogen Peroxide and Organic Peroxides on Quantum- sized Semiconductor Colloids. Environmental Science and Technology 28:776-785.
- Anandan S, Vinu, A, Venkatachalam N, Arabindoo B, Murugesan V (2006) Photocatalytic activity of ZnO impregnated Hß and mechanical mix of ZnO and Hß in the degradation of monocrotophos in aqueous solution. Journal of Molecular Catalyses A: Chemical 256:312-320.
- Sharma VK, Dutta PK, Ray AK (2007) Review of kinetics of chemical and photocatalytical oxidation of Arsenic(III) as influenced by pH. J Environ Sci Health A Tox Hazard Subst Environ Eng 42: 997-1004.
- Mondal K, Sharma A (2014)Photocatalytic Oxidation of Pollutant Dyes in Wastewater by TiO2 and ZnOnano-materials–A Mini-review. Department of Chemical Engineering, Indian Institute of Technology, Kanpur, India pp. 36-72
- Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170: 520-529.
- Sharma VK (2009) Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—A Review. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering 44:1485-1495.
- Montazerozohori M, Nasr-esfahani M, Joohari S (2012) Photocatalytic degradation of an organic Dye in some aqueous buffer solutions Usingnano titanium dioxide: a kinetic study. Environment Protection Engineering 38:45-55.
- Epifani M, Comini E, Díaz R, Genç A, Terreca AJ, etal. (2015) Surface modification, hetrojunctions, and other structures: composing metal oxide nanocrystals for chemical sensors Oxide-based Materials and Devices VI, SPIE proceeding, 936415: doi:10.1117/12.2078017.
- Enachi M, Lupan O, Braniste T, Sarua A, Chow L,et al. (2015) Integration of individual TiO2 nanotubes in the chip: Nanodevice for hydrogen sensing. Physica Status Solidi – Rapid Research Letters 9:171-174
- Tiwari D, James SW, Tatam RP, Korposh S, Lee SW (2015) A high sensitive chemical sensor based on titania coated optical fiber long period grating for ammonia sensing in water, Proc. SPIE 9655, Fifth Asia Pacific Optical Sensors conference 9655.
- Tian WC, Ho YH, Chen CH, Kuo CY (2013) Sensing performance of precisely ordered TiO2 nanowire gas sensors fabricated by electron-beam lithography. Sensors (Basel) 13: 865-874.
- Troy KT, Erwin M, Sabio N, Browning D, Frank EO (2011) Photocatalytic water oxidation with suspended alpha-Fe2O3 particles-effects of nanoscaling. Energy and Environmental Science 4:4270-4275.
- Wei Q, Zhang Z, Li Z, Zhou Q, Zhu Y (2008) Enhanced photocatalytic activity of porous-Fe2O3 films prepared by rapid thermal oxidation. Journal of Physica D: Applied Physics 41:20200-202002.
- Karunakaran C, Senthilvelan S (2006) Electrochemistry Communications 8:95-101.
- Vladimir MA, Valeri MA, Gohar ES, Mikayel SA,et al. (2015) The ethanol sensors made from a-Fe2O3 decorated with multiwall carbon nanotubes. Advances in Nano Research 3:1-11.
- Wang Y, Guiyun Yi, Kai L, Sun G, Wang Xiaodong B,et al. (2015) Synthesis, Characterization and Gas Sensing Properties of Ag-doped a-Fe2O3 by Solid-state Grinding Method. Current Nanoscience 11:419-423.
- Zhang A, Zhang J (2010) Visible-light activities of Gd2O3/BiVO4 composite photocatalysts. Journal of Materials science 45:4040-4045.
- Abdullah MM, Rahman MM, Faisal M, Khan SB, Singh P,et al. (2014) Fabrication of Ethanol Chemical Sensors Based on As-Prepared Gd2O3Nanorods by Facile Hydrothermal Routes. Journal of Colloid Science and Biotechnology 2:1-6.
- Abdullah MM, Rahman MM, Bouzid H, Faisal M, Khan SB,et al. (2015) Sensitive and fast response ethanol chemical sensor based on as-grown Gd2O3 nanostructures. Journal of Rare Earths 33:214-220.
- Jamal A, Raahman MM, Khan SB, Abdullah MM, Faisal M, et al. (2013) Simple Growth and Characterization of a-Sb2O4: Evaluation of their Photo-catalytic and Chemical Sensing Applications. Journal of the Chemical Society of Pakistan 35:570-576.
- Tyagi S, Aggarwal H, Ikram S (2010) A Dynamic Electrochemical Sensor For Europium. International journal of chemistry 49:1325-1331.
- Agarwal H, Sharma D, Sandhu SK,Tyagi S, Ikram S (2010) Removal Of Mercury From Waste Water- Use Of Green Adsorbents – A Review. Electronic Journal of Environment Agriculture & Food Chemistry 9:1551-1555.
- Tyagi S, Agarwal H, Ikram S (2011) Uranyl Selective Polymeric Membrane Sensor based on p-tert-butylbiscalix[4]arenes. Analytical &Bioanalytical Electrochemistry 3: 350-364.
- Tyagi S, Agarwal H, Ikram S (2011) A Polymeric Membrane Electrode Based on p-tert-butylthiacalix[4]arene Derivative for Thorium Determination. Analytical &Bioanalytical Electrochemistry 3:436-449.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences