Theoretical Calculation for Removing Tau Protein in Alzheimer Disease
Behrouz AH, Zareyi H, Zhour K, Tashakori SH and Vaezzadeh M
DOI10.21767/2471-9838.100035
Faculty of Science, Department of Physics, K.N. Toosi University of Technology, Tehran, Iran
- *Corresponding Author:
- Zareyi H
Faculty of Science, Department of Physics
K.N. Toosi University of Technology
Tehran, Iran.
Tel: +982123064455; E-mail: zareyi.h@mail.kntu.ac.ir
Received date: July 13, 2018; Accepted date: September 03, 2018; Published date: September 06, 2018
Citation: Behrouz AH, Zareyi H, Zhour K, Tashakori SH, Vaezzadeh M (2018) Theoretical Calculation for Removing Tau Protein in Alzheimer Disease. Nano Res Appl Vol.4 No.2:6. doi:10.21767/2471-9838.100031
Copyright: © 2018 Behrouz AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Alzheimer's disease (AD) is the most prevalent form of dementia, which affects people older than 60 years and cause suffering for more than 44 million over the world. Plaques formation is one of the first step in the treatment of this illness. The elimination of the accumulation of these plaques by using the resonance oscillation and heat production of ultrasound’s bubble can reduce the side effect of the neurodegenerative disease. In this paper, the resonance frequency is calculated precisely as a function of the required radius, which is the same as the radius of the plaques in the brain. The results are in good agreement with experimental data and just have a negligible percentage error, less than 0.67%. These results can help in modeling accurate frequency for mechanical treatment before in vivo treatment which can reduce the harmful side effects and other healthy cells damage.
Keywords
Alzheimer's disease; Tau protein; Bubble; Ultrasound; Resonance frequency
Introduction
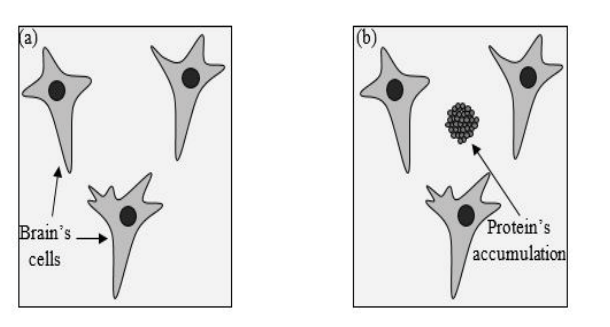
The Alzheimer disease was first described by Alois Alzheimer [1]. This illness is processed by formation of the plaques in the brain of the patient [2,3]. Many various methods have been presented for treating this disease. In spite of the big effort in understanding this illness, few effective treatments and cures had been reported [2]. The recent proposals for solving this problem is not only a biological method, but also it takes its way to physics. One of the most important dilemmas that has been faced is the impermeability of the Blood-Brain Barrier (BBB) [4]. Recently, bubbles produced by focused ultrasound wave bring new methods for applying safe, effective, noninvasive and local penetration in the brain. These bubbles contribute in drug delivery and removing the plaques [5]. The formation of the aging plaques is the most crucial factor in appearing the AD. These plaques form the concentration of Amyloid Beta (Aβ) Peptide [6]. Destructive cooperation of these plaques starts from the Hippocampus that is responsible to form memories, [7,8] as a consequence the loss of temporary memory will be the first sign of this disease. After that, the mutated proteins will attack the other parts of the brain increasingly leading to a specific alteration of the disease process. The effect of these proteins is related to the location of their gathering place in the brain, for example if the proteins are accumulated in the front part of the brain, the proteins will annihilate logical thinking ability and then the feeling control part which cause an extreme alteration in temperament of the patient, while the accumulations on the upper part can cause illusion and paranoia. If the plaques reach to the back of the brain, they remove the deepest memories of the patient forever. The main reason of neural string contortion in the AD is Tau protein. Brain neural cells have passages network that carries molecules supply. In conditional mode, Tau protein’s role is to make sure this passage works properly, but in the of AD, Tau proteins will be trapped in the contortion strings and the passages will collapse. Consequently, scientists declare that AD is caused by deposition of Aβ in plaques in brain tissue as shown in Figure 1.
Finding a harmless way for opening the strings is essential. One of these ways is applying ultrasound waves. Ultrasound waves are used for scanning inside the body, by using this high frequency sonic waves physician are able to create images of various part inside the body which is very helpful detecting diseases inside the human body, and actually they may help in the treatment of Alzheimer's disease [9]. However, the main problem is that a thin shield of cells, called Blood Brain Barrier (BBB), protects the brain and it is very difficult to be crossed by things like therapeutics or helpful antibodies. Recent studies showed that focused sound waves open the blood-brain barriers and allow the delivery of drugs to the damaged cells in order to achieve again a normal function of brain cells [10]. Since this method has been the only way to produce reversible BBB disruption without brain tissue damage, it has been extensively investigated by several research groups.
Focused ultrasound waves have the ability to destruct precisely, a predetermined volume of a chosen tissue without affecting to other surrounding tissues [11]. This technique is improved in recent decades to increase the precision and reduce the side effects [12]. The ablation with ultrasound and enhancing ultrasound heating effect are important applications of ultrasound treatment [13,14]. This method generates cavitation for heating to match the cavitation clouds with the heating patterns for treatment [15] especially for brain disease and neurological purpose [16,17].
Combining ultrasound with harmless microscopic bubbles, as ultrasound contrast agents, could be an effective detecting technique. In addition, the sound waves cause the bubble to vibrate and expand, which cause friction then explosion that lead to mechanical impact on the surrounding [18]. The increasing temperature generated in the ambient due to the friction affects the cell membrane and the Tau proteins, which can lead to destroy them.
The purpose of this work is to understand the mechanical effect of ultrasound reacting with the surrounding and to investigate its appropriate frequency [19] by modeling and mathematics, because it is the way for better illustration of the methods before treatments. Physical characteristics of biological tissue enforce bubble formation during the propagation of ultrasound wave, this case can also be studied by modeling and simulation [20-22].
Because of the bubbly shape of these proteins, this model is based on bubble formation, and then these mechanical effects are considered. Moreover, the effect of the pressure on resonance frequency has been investigated in order to achieve more accurate results. This model helps in treating the diseases with the less side effect and calculate the more convenient frequency to deal with the situation. This paper focus on the experimental study accomplished by Leinenga [19] and coworkers to obtain an accurate theoretical model that it based on their work. Our calculation is about a bubble model surrounded by liquid.
Models and Equations
Minnaert M showed that the bubbles formed when air escapes from an orifice within a flu would give sound by pulsation, because each pulse makes a small cavity in the water that it makes a sound when closed [23,24]. In this model, the rigid walls of the bubbles act as a resonator. Therefore, it has a frequency and it has been determined as:
 (1)
(1)
Where a is the bubble radius, P is the ambient pressure, γ is an adiabatic index for water and ρ is the density of the ambient [24].
The experimental data for bubble-lipid shell and Tau protein have been obtained from Leinenga and co-workers work [19]. In this study, we considered 4 μm for bubble radius, 1.3 for adiabatic index in 1 atm pressure, 1.35 g/cm3 for density. By replacing these values in equation (1) we can obtain that the accurate frequency:
f = 0.676×106 Hz
which is very close to the experimental data that was reported as 0.7 MHz. The relative error percentage for this calculation is about 0.13%.
For more accurate investigation another formula is considered, which is one of the best methods for describing the ultrasonic waves in liquid compressibility, this formula was presented by Keller [25]. This study focuses on Taus proteins, which belongs to a group of Microtubules-Associated proteins. For modeling in this situation disc-shaped treatment of Aβ folding is considered [26].
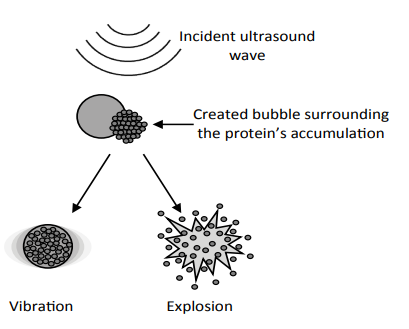
In order to apply ultrasound treatments, micro bubbles must be developed. These bubbles are gas trapped in a lipid shell. The bubble will contract at the frequency of the propagating acoustic wave when it passes through the tissue that exposed to ultrasound, because of the cyclic pressure radiation. Consequently, the bubble will start to oscillate and forces the surrounding fluid to flow, where the force of the spreading wave is radial. When the bubble oscillation amplitude becomes so large, and after exceeding a specific threshold, the bubble will collapse due to the stress applied by the surrounding fluid, consequently a shock wave will propagate at supersonic speed radially from the collapsed site which lead to the production of high temperature and the increase of pressure dramatically.
The frequency of the incident ultrasound wave creates a bubble that has the same dimension of protein’s accumulation and at the same time it causes a resonance vibration of the created bubble. This vibration lead to increase the temperature due to friction which leads to damage the Aβ protein. On the other hand, the bubble can collapse under effect of the shock wave introduced by the ultrasound wave which lead to a mechanical impact and consequently the explosion of the accumulation (Figure 2).
The acoustic field pressure equation (2) at the location of the bubble will be considered as a sinusoidal function. P(t) is the ultrasound pressure driving modeled as a spatially homogeneous standing sound wave with sinusoidal function:
 (2)
(2)
Where pa is the pressure amplitude that is about 1 to 10 atm and ωd is the driving angular frequency. Recent works have shown that many features of the bubbles can be expressed within a hydrodynamic approach [27], especially the bubbles stability dependence on experimental parameters. Furthermore, the analysis of bubble wall dynamics gives many important properties. These dynamics are conventionally described by the Rayleigh- Plesset equation. A lot of improvement had been presented for the modeling of the spherical domain walls dynamics in liquids, after Lord Rayleigh modeled the collapse of an empty cavity in a liquid. Plesset presented the main step. This step declares the expression of a variable external driving pressure and the effect of surface tension on the bubble dynamics (1949).
Navier-Stokes equations are utilized for the bubble radius calculation with ordinary differential equation (ODE) [28]:
 (3)
(3)
where P(R,t) is the pressure on the liquid side of the interface, P(t)
is the imposed acoustic field pressure evaluated at the location of
the bubble, P0 is the static ambient pressure,  is the speed of the
bubble wall, Cwis the sound speed of the liquid and ρw is the lipid
density, in this case the surface tension, σ, will take the value of
1.35 g/cm2 [29].
is the speed of the
bubble wall, Cwis the sound speed of the liquid and ρw is the lipid
density, in this case the surface tension, σ, will take the value of
1.35 g/cm2 [29].
The bubble moves slowly with respect to the sound velocity in the gas. The pressure in the gas, throughout the bubble, is uniform. In this case the pressure depends on the bubble volume and the heat transfer across the bubble wall which is produced by friction due to the bubble oscillation [30]. The calculation of the pressure across and near the bubble wall can be given by adopting this relation as a simplified model:
 (4)
(4)
Which is assumed to obey van der Waals type process equation, where R0 being the ambient bubble radius, h is the hard-core van der Waals radius andP0is 1 atm [31]. It is necessary to mention that Equation (4) presupposes homogeneity of the pressure inside the bubble. “h” is given by the following relation.
 (5)
(5)
Since the velocity of sound in water is high, the differential part of the Rayleigh-Plesset equation can be disregarded. And since the radius is large, R3 >> h3 , the excluded volume h3 can be neglected in the Van Der Waals formula, therefore Equation (2) can be replaced by the ideal gas law under isothermal conditions. Considering the resonance condition, the bubble radius will reach its maximum value. Therefore, we can consider that R (t) =R0 (1+x(t)) and by replacing it in equation (3) the resonance frequency can be obtained. The conventional elastic pendulum model had been used in this equation where the oscillations take place along the X direction.
 (6)
(6)
Equation (6) is rewritten as below:
 (7)
(7)
As mentioned before R3»H3, so we take R3 - h3![]() R03 and Equation (7) could be rewritten as:
R03 and Equation (7) could be rewritten as:
 (8)
(8)
Therefore, the following equation can be obtained:
 (9)
(9)
Furthermore, all used equations are for standing waves created in finite volume. The initial value of bubble radius is assumed to be between 1 and 5 μm and the sound speed in water is 1481 m/s [32].
By integrating Equation (9) with respect to time, the velocity of the walls is obtained:
 (10)
(10)
For x=0 and Pa =0, and by eliminating all the squared x and its derivatives terms in equation (9).
 (11)
(11)
Where γ = 2ʋ/R02 is the damping factor for the lipid shells.
 (12)
(12)

With R0 = 4 × 10-6 (unit) and k=1.3 (unit) and the resonance frequency by regarding damping factor would be:
 (13)
(13)

Therefore, the error percent would be:

This result shows good agreement with experimental data for Alzheimer treatment which has been obtained by Leinenga and his coworker [19]. In addition, the prediction of conditions for other cases and applications will be used before an operation and surgery. Therefore, these calculations are useful for physicians and scientist who involve with similar cases for removing protein's disorder.
Conclusion
In this work, a model was expanded to predict the accurate frequency for the predetermined radius and the convenient frequency of ultrasound bubble for the treatment of the Alzheimer disease. These bubbles can help in removing the Amyloid-Beta, which aggregates in the brain and cause disorder intercellular interaction. The obtained results are in good agreement with experimental data and just have a negligible percentage error that is less than 0.67%. This can bring a new calculation for pretreatment of Alzheimer. The application of this frequency model before in vivo treatment leads to reduce the harmful side effects and other cell damage, because its mechanical effect focuses only on the abnormal section in the brain.
Future scope
Safety issues of ultrasound is major challenges for the ultrasound-based strategies translation for the neurological diseases prevention and treatment by helping modeling and predetermined pattern of treatment is the future purpose of this work.
References
- Goedert M, Spillantini MG (2006) A century of Alzheimer's disease. sci 314: 777-781.
- Garcia AM, Sisternas A, Hoyos SP (2008) Occupational exposure to extremely low frequency electric and magnetic fields and Alzheimer disease: A meta-analysis. Int J Epidemiol 37: 329-340.
- Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, et al. (1985) Neuronal origin of a cerebral amyloid: Neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J 4: 2757.
- Konofagou EE, Yao Sheng Tunga, James Choia, Thomas Deffieuxa, Babak Baseria, et al. (2012) Ultrasound-induced blood-brain barrier opening. Curr Pharm Biotechnol 13: 1332-1345.
- Hynynen K (2008) Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev 60: 1209-1217.
- Sadigh ES, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, et al. (2015) Amyloid-beta: A crucial factor in Alzheimer's disease. Med Princ Pract 24: 1-10.
- David JP, Farida Ghozali, Catherine Fallet Bianco, Annick Wattez, Stephanie Delaine, et al. (1997) Glial reaction in the hippocampal formation is highly correlated with aging in human brain. Neurosci Lett 235: 53-56.
- Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci 12: 383-388.
- Raymond SB, Lisa HT, Jonathan DD, Nathan JM, Kullervo Hynynen, et al. (2008) Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PloS one 3(5): e2175.
- Kinoshita M, Nathan McDannold, Ferenc AJ, Kullervo Hynynen (2006) Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 340: 1085-1090.
- ter Haar G, Sinnett D, Rivens I (1989) High intensity focused ultrasound-a surgical technique for the treatment of discrete liver tumours. Phys Med Biol 34(11): 1743.
- Cheng C, Xiao Z, Huang G, Zhang L, Bai J (2017) Enhancing ablation effects of a microbubble contrast agent on high‐intensity focused ultrasound: An experimental and clinical study. BJOG 124: 78-86.
- Bastianpillai C, Neophytos Petrides, Taimur Shah, Stephanie Guillaumier, Hashim UA, et al. (2015) Harnessing the immunomodulatory effect of thermal and non-thermal ablative therapies for cancer treatment. Tumor Biol 36: 9137-9146.
- Liu YJ, Lin Xue Qian, Dong Liu, Jun Feng Zhao (2017) Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med 242: 1515-1523.
- Natarajan S, Tonye AJ, Alan MP, Rory Geoghegan, Patricia Lieu, et al. (2017) Focal laser ablation of prostate cancer: Feasibility of magnetic resonance imaging-ultrasound fusion for guidance. J Urol 198: 839-847.
- Goto K, Ryo Takagi, Takuya Miyashita, Hayato Jimbo, Shin Yoshizawa, et al. (2015) Effect of controlled offset of focal position in cavitation-enhanced high-intensity focused ultrasound treatment. Japanese J Appl Phys 54: 07HF12.
- Leinenga G, Christian Langton, Rebecca Nisbet, Jürgen Gotz (2016) Ultrasound treatment of neurological diseases: Current and emerging applications. Nat Rev Neurol 12: 161.
- Tanzi R, Moir R, Wagner S (2004) Clearance of Alzheimer's Aβ peptide: The many roads to perdition. Neuron 43: 605-608.
- Leinenga G, Götz J (2015) Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci Transl Med 7: 278ra33.
- Curra FP, Mourad PD, Khokhlova VA, Cleveland RO, Crum LA (2000) Numerical simulations of heating patterns and tissue temperature response due to high-intensity focused ultrasound. Ieee T Ultrason Ferr 47: 1077-1089.
- Church CC, Labuda C, Nightingale K (2015) A theoretical study of inertial cavitation from acoustic radiation force impulse imaging and implications for the mechanical index 1. Ultrasound Med Biol 41: 472-485.
- Huang C, Sun MK, Chen BT, Shieh J, Chen CS, et al. (2015) Simulation of thermal ablation by high-intensity focused ultrasound with temperature-dependent properties. Ultrason Sonochem 27: 456-465.
- Ammari H, Brian Fitzpatrick, David Gontier, Hyundae Lee, Hai Zhang (2016) Minnaert resonances for acoustic waves in bubbly media. Cornell University Library.
- Minnaert M (1933) On musical air-bubbles and the sounds of running water. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 16: 235-248.
- Keller JB, Miksis M (1980) Bubble oscillations of large amplitude. J Acoust Soc Am 68: 628-633.
- Kolarova M, Francisco García Sierra, Ales Bartos, Jan Ricny, Daniela Ripova (2012) Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis pp: 1-3.
- Hoomans B, Kuipers JAM, Briels WJ, Van Swaaij WPM (1996) Discrete particle simulation of bubble and slug formation in a two-dimensional gas-fluidised bed: A hard-sphere approach. Chem Eng Sci 51: 99-118.
- Hilgenfeldt S, Michael P, Siegfried Grossmann, Detlef Lohse (1998) Analysis of Rayleigh–Plesset dynamics for sonoluminescing bubbles. J Fluid Mech 365: 171-204.
- Scanu A (1966) Forms of human serum high density lipoprotein protein. J Lipid Res 7(2): 295-306.
- Brenner MP, Hilgenfeldt S, Lohse D (2002) Single-bubble sonoluminescence. Rev Mod Phys 74: 425.
- Prosperetti A, Hao Y (1999) Modelling of spherical gas bubble oscillations and sonoluminescence. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 357: 203-223.
- Hu X, Crick SL, Bu G, Frieden C, Pappu CRV, et al. (2009) Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proceedings of the National Academy of Sciences 106: 20324-20329.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences