A New Type and Effective Approach for Anti-Cancer Drug Delivery Application: Nanosponge
Bergal A, Elmas A, and Akyüz G
Department of Nanoscience and Nanotechnology, Graduate School of Applied Sciences, Ondokuz Mayıs University, Atakum, Samsun, Turkey
- *Corresponding Author:
- Bergal A
Department of Nanoscience and Nanotechnology
Graduate School of Applied Sciences
Ondokuz Mayıs University-55200 Atakum, Samsun, Turkey
Tel: +905327984165
E-mail: ayber2002@yahoo.com
Received Date: December 10, 2019; Accepted Date: December 23, 2019; Published Date: December 30, 2019
Citation: Bergal A, Elmas A, Akyüz G (2019) A New Type and Effective Approach for Anti-Cancer Drug Delivery Application - A Nanosponge. Nano Res Appl Vol.5 No.3:1
DOI: 10.36648/2471-9838.5.3.43
Abstract
The delivery of anti-cancer drugs to the desired or targeted region is one of the most critical and difficult problems faced by today's researchers. Therefore, cyclodextrin-based nanosponge (CD-NS) plays an important role in scientific research on the guided release of anti-cancer drugs. Many anti-cancer drugs with both lipophilic and hydrophilic properties can be integrated or loaded into Nanosponge (NS) for targeting purposes, and ultimately the solubility and the bioavailability of the drug is increased while protecting from environmental influences. NSs prepared by reacting cyclodextrins (CD) with cross-linkers such as carbonyl-diimidazole, diphenyl carbonate, hexamethylene diisocyanate and pyromellitic anhydride are nanosize, cross-linked, non-toxic, porous and stable in high temperature polymers. Having to spherical shapes, swelling properties and porous structures, as well as biologically safe and biodegradable NSs offer higher drug loading than other nano carriers. The main advantages of NS as a nanocarrier in drug delivery systems are due to ability to bind weakly soluble drugs in their three dimensional matrix, increase their bioavailability and the aqueous solubility of water-soluble molecules, protect the degradable substances in their structure and controlled release. NSs can also be used as subsidiary in the preparation of pellets, capsules, tablets, suspensions, solid dispersions, granules or topical dosage forms. In this review article, the importance of CD-NS has been emphasized in terms of showing its properties, preparation methods, a new generation of nanocarrier drug delivery agent and therapeutic applications.
Keywords
Nanosponge; Guided drug delivery; Cancer; Controlled drug delivery; Cyclodextrin; Nanocarriers
Introduction
Cancer is simply a group of diseases with abnormal cell growth that has the potential to spread to other parts of the body and has lost its program and function to know what to do and when to do compared to a healthy cell. One of the most common strategies for cancer treatment is surgery, radiation therapy, chemotherapy, hormone therapy, and targeted therapy using anti-cancer drugs.
Most drugs currently used for the treatment of cancer are not targeted and have poor bioavailability and poor biopharmaceutical properties [1] that limit their use in clinical applications, low permeability, short blood circulation time and/or high molecular weight causing various toxic side effects. This results in rapid excretion, deterioration of gastrointestinal fluids with nonspecific toxicity, in vitro stability, strong dose-dependent side effects, and lack of selectivity [2]. To overcome these disadvantages of traditional therapeutic approaches and to solve these problems targeting drug especially nano-carrier based delivery systems have been extensively researched and developed.
The main function of the nanocarrier (NC) is designed to deliver therapeutic drugs to the targeted area. It is important that the nanomaterials used for this purpose are soluble, safe and biocompatible and bioavailable [3-5]. NC does not obstruct blood vessels and the toxicity values of nanomaterials used for drug delivery should be very low, so that they can be used to target specific diseased tissue at a safe concentration [6].
Literature Review
Nanocarrier drug delivery systems
To design an effective NC, it is necessary to understand the unifying effects of size, shape, surface chemistry, patientspecific information and other parameters. It is important that the ideal carrier is safe, efficient and has optimum bioavailability as well as high stability, non-mutagenic and nontoxic and ability to target to a specific site. For this purpose, many nanoparticulate systems such as nanosponges, liposomes, dendrimers, micelles, inorganic structures, nanotubes, nanocrystals, hydrogels, magnetic nanoparticles, microspheres and microcapsules [7] have been developed, designed and manufactured.
However, designing an ideal drug delivery system makes it difficult to achieve the desired results due to the disadvantages of nanocarriers. For example, liposomes have low drug loading content, low stability, high cost, and undesirable hydrophobic drug release. It limits the application of some nanomaterials as a drug delivery system due to their non-degradability or toxicity properties in body tissue.
Methods to transport drug-loaded nanoparticles
Delivering of drug-loaded nano-carriers to the target site can be achieved by 3 different mechanisms: passive, active and physically targeting. Passive targeting leads to an increased permeability and retention (EPR) effect, which allows tumor cells to absorb preferably NC-sized bodies [8]. Although most tumors vary in pore size depending on tumor type, they are reported to have much larger pores (about 380_780 nm) than healthy organs [9,10]. Therefore, the size of the prepared nano structure is important for the accumulation of the tumoral region by the effect of EPR in the vessel walls and permeability to the tumor vascular system [11].
The active targeting approach is a method of conducting surface modifications to nanocarriers using certain ligands such as antibodies, sugars, folic acid, peptides, and introducing and binding ligand nanostructures to a particular tumor cell receptor [12,13]. Physical targeting is a method for directing NCs to the target site and controlling the release process using external sources or areas, for example photothermal and magnetic field [14].
Once an ideal anti-cancer drug nanocarrier is designed and the targeting method is determined based on tumor structure and environmental influences, release of the cancer drug at the selected target site can be triggered from the nanocarrier by an internal or external stimulatory response mechanism. Systems that respond to internal stimuli are based on environmental differences between diseased and normal tissue.
Internal stimuli can be with temperature [15], pH gradients [16], redox potential [17,18] and enzymes in the system as a result nanocarrier designed according to one of the internal stimuli systems makes drug release. Light [19], ultrasound [20], electric field [21], magnetic field [22] voltage excitations [23] and other chemicals [24] as external stimulants that drug is released by external effects.
Since drug release can be stimulated by environmental changes, the increase and decrease in swelling characteristics of these substances are very useful for drug release applications [25]. Among these, pH-sensitive systems are widely applied in cell organelles, tissues or organs to selectively release cancer drugs to achieve better efficacy and avoid side effects [26].
Cyclodextrin based nanosponges (Cd-Nss)
Nanosponges is one of the nanocarriers that the researchers recently have been working on as a drug delivery agent designed to improve the efficacy of eliminating the disavantases of conventional drugs for the reasons described in detail above. Before elaborating on this concept, it is useful to briefly mention one of the main components, cyclodextrins.
Cyclodextrins (CD's)
Properties of cyclodextrins: Cyclodextrin (CD) which consists of enzymatic decomposition of starch, one of the most basic polysaccharides in nature, by Bacillus amylobacter [27] bacteria and composed of the binding of Dglycosupranoses with α-1,4-glycosidic bonds (Figures 1a and 1b). Those are water soluble, biocompatible and have lipophilic cavity and hydrophilic outer surface. Since the 1970s, it has been used in the fields of medicine, food, cosmetics, textile, catalyst, biotechnology as well as a drug delivery system in nanotechnology, pharmacology and pharmaceutical industry [28].
Chemical structure of CDs: They are named α-CD, β-CD and γ-CD according to the number of glycosupranose units in their structure. For example, the structure consisting of the binding of 6 glycosupranose units by glycosidic bonds is defined as α- CD, the structure consisting of binding of 7 glycosupranose units β-CD and the structure consisting of binding of 8 glycosupranose units called (γ-CD) (Figure 1b). Although it is present in nature in higher bound structures, it is not preferred because it is very difficult to obtain and can form very few inclusion complexes with many substances. Among these, β- CD has been preferred as the most studied structure because of its complex cavity with many organic and inorganic materials, high drug loading capacity, easy availability and cheap price [29].
The CD molecule are shaped into a truncated cone or funnel with two open ends due to the sequence of glycopyranose units, with primary hydroxyl groups at one end and secondary hydroxyl groups at the other end (Figure 1c ).
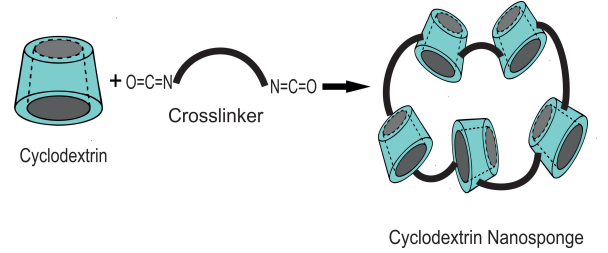
Concept of cyclodextrin nanosponges (CD-NSs): Natural CDs cannot form coverage complexes with hydrophilic or high molecular weight drugs, however, some natural CDs may exhibit toxic properties when injected intravenously [30-32]. Therefore, in order to overcome the limitations of natural CDs and improve their properties, many chemical modification studies have been conducted to increase their physicochemical properties and to use them as a nanotechnological drug delivery system. One of them is the formation and development of NSs by the reaction of natural CDs with crosslinker s, and that NSs can be applied as a nano-drug release system has been first time demonstrated by Trotta et al. [33].
Nanosponges are hyper-crosslinked cyclodextrins having nanoscale spongy pores and a three-dimensional network, which can be obtained with a certain amount of α, β and γ cyclodextrins crosslinked with an appropriate amount of crosslinking agent. Therefore, they can keep the hydrophobic and hydrophilic drugs in their cavity. NSs are generally prepared from β-cyclodextrins, because among natural (α, β and γ) CDs, β - CD has the highest complexity and stability due to the appropriate cavity size with crosslinkable polymers.
Briefly, NSs are non-toxic high temperature stable structures [34,35] prepared by reacting cyclodextrin (β-CD) with crosslinking polymer such as carbonyl-diimidazole, diphenyl carbonate, hexamethylene diisocyanate and pyromellitic anhydride (Figure 2).
Compared with natural CD, they form higher sites of interaction with drugs and higher drug encapsulation complexes. NSs are being explored as a promising nanocarrier system to improve drug solubility, prevent drug degradation, increase permeability, and control drug release [35]. For this purpose, paclitaxel, camptothecin, quercetin, telmisartan, meloxicam, tamoxifen, nifedipine, erlotinib, acetylsalicylic acid, captopril, ibuprofen, lansoprazole and enalapril are used as a nanocarrier for various drugs [36-39].
Cyclodextrin polymer with different cross-linkers: Although there are different types of cross-linker s, Nanosponges are synthesized using four main types of cross-linking polymer materials, such as carbonyl cross-linker s, diisocyanate linkers, anhydride cross-linkers, and epichlorohydrin. A brief summary of the cross-linker s used is summarized below (Figure 3).
Carbonate nanosponges
Carbonate Nanosponges containing carbonate bonds between two cyclodextrin monomers can be prepared by solvent extraction, thermal desorption or microwave and ultrasound assisted synthesis methods using carbonyl crosslinker s such as diphenyl carbonate, carbonyl imidazole, and dimethyl carbonate (Figure 3).
They may be amorphous or crystalline according to the synthesis methods used. For example, by using the melting method, a crystalline structure is obtained, while using the solvent method an amorphous nanosponge can be obtained. Various drugs such as paclitaxel, tamoxifen, reservertol, telmisartan, curcumin, itraconazole, 1-DOPA, camptothecin, erlotinib, and quercetin are available with this type of nanosponge [40-44].
Carbomat nanosponges
Such NS prepared by solvent method under an anhydrous/ nitrogen atmosphere at room temperature to 70°C is obtained by reacting cyclodextrins with cross-linkers such as hexamethylene diisocyanate and toluene diisocyanate in a molar ratio of 1:2 to 1:8 (Figure 3). Originally synthesized by DeQuan Li and Min Ma [45,46] in 1998, such NSs were developed for the treatment of water, including the removal of dissolved organic carbon such as nitrophenol. The last step is to prepare molecularly imprinted nanospongs for the encapsulation of substances such as steroids, dyes and dextromethorphan using diisocyanate cross-linker s [47-49].
Anhydride nanosponge
Anhydride Nanosponge can be prepared by the solvent method in the presence of a base such as pyridine or triethylamine to accelerate the polymerization by using crosslinkers such as pyromellitic dianhydride ethylene-diaminetetra- acetic acid dianhydride of the cyclodextrins at room temperature (Figure 3). Cyclodextrin: cross-linking molar ratios ranging from 1: 2 to 1: 8 are used for preparation. Numerous studies have been conducted to encapsulate various drugs such as doxorubicin, meloxicam, ibuprofen and acetylsalicylic acid with this type of NS [50-52].
Epichlorohydrin cyclodextrin nanosponges
These NSs prepared by dissolving cyclodextrins in a basic medium such as sodium hydroxide using cross-linking agents such as epichlorohydrin (Figure 3) are more hydrophilic in nature. Such nanospongs which exhibit high chemical resistance and adjustable swelling capability have been used to encapsulate drugs such as creatinine and captopril, enalapril, silazapril, but in some studies a cross-linking molar ratio of up to 1:10 with cyclodextrin has been studied [53].
Nanosponge preparation methods
The most commonly used NSs synthesis method can be briefly described as dissolving any selected type of CD in a suitable solvent, then adding a catalyst if necessary and finally adding a desired type of cross-linker under continuous mixing or sonication [54,55]. In some cases, increasing the temperature to initiate the crosslinking process may also involve melt polymerization if the crosslinking agent is in liquid form and dissolves CDs [56].
Techniques used in the synthesis of cyclodextrin based nanosponges
Melt method: In this method, the CD is heated and reacted at 100°C on a magnetic stirrer for 5 hours with excipients such as selected cross-linker, solvent such as dimethylformamide (DMF) or reaction accelerator catalyst. The product brought to room temperature is decomposed and washed to remove unreacted basic components and formed by-products using a suitable solvent (such as ethanol) [55]. Purification is the most critical and important step in this method since the byproducts of different structure will be formed according to the type of cross-linker used and the toxicity of the by-products may remain in the final product.
Solvent method: According to this method, CD (usually β – CDs) is mixed with a polar aprotic solvent, in particular dimethylsulfoxide or dimethylformamide (DMSO/DMF), and carbonyl compound cross-linker such as diphenyl carbonate (DPC), dimethyl carbonate (DMC) or carbonyldiimidazole (CDI) is added to optimize the process based on the cross-linker/ polymer molar ratio. The reaction is carried out at temperatures ranging from 10 c to reflux for 1 to 48 hours. The melting step is eliminated compared to the solvent method. After allowing cooling to room temperature, an excess of purified water is added to remove unreacted products and the product is then filtered off under vacuum.
The product is purified by prolonged soxhlet extraction with ethanol for complete recovery and further purification [56,57]. The product is then dried and milled in a mechanical mill to prepare a homogeneous powder. The product obtained is solid nanoparticles with high dissolution power for molecules that are slightly soluble in water with a spherical morphology.
Ultrasound assisted synthesis: In this method, Nanospongs are described in Trotta et al. [55] can be synthesized spherically and uniformly under 5 microns using the ultrasonication technique according to the method employed. NSs are obtained by reacting CDs with cross-linkers such as diphenyl carbonate (DPC) or pyromellitic dianhydride (PMDA) under sonication in the absence of solvent.
According to the method of Trotta et al. [55], anhydrous β- CD and DPC are mixed in a 250 ml capacity flask in the absence of a specified molar ratio of solvent. The flask is then placed in an ultrasound bath (filled with water) and heated at 90°C and sonicated for 5 hours. The mixture is allowed to cool and then ground in a mortar and purified first by distilled water to remove unreacted polymer, then by Soxhlet extraction with ethanol for further purification. The product is dried under vacuum and stored at 25°C for subsequent use. The main advantages of this method are that it can be replaced by processes with a high energy input, such as probe sonication, and that no organic solvents are used.
Microwave assisted synthesis: Microwave-assisted synthesis, a simple method for synthesizing cyclodextrin-based nanospongs, is one of the most important advantages compared to other methods in that it has a four-fold reduction in reaction time, high crystallinity, homogeneous particle size distribution and homogeneous crystallinity [58].
General application of cyclodextrin nanosponges
Due to their versatile structure with nanopore, they can carry both hydrophilic and hydrophobic drugs, increase their satiability and dissolution rate in aqueous media and have the ability to prevent the deterioration of drugs. In terms of their controlled release, biocompatibility, NSs has many potential applications in the pharmaceutical field, in particular targeting drugs in the treatment of tumors and cancer [59,60]. The EU commission report states that NSs can be used as a promising, innovative system for drug transport [61,62].
One of the most critical problems encountered in the development of new drugs is the low solubility of the drug in aqueous media. Because the drug taken orally should be in solution form at the site of action to be absorbed from the gastrointestinal tract (GIT) in the systemic circulation. Therefore, given the characteristic structures and properties of nanosponges, the ability to form inclusion complexes with drugs can be widely used in the pharmaceutical field to increase the solubility of molecules that have very low solubility in water, and hence to increase the bioavailability of lipophilic drugs.
Swaminathan et al. [63] aimed to increase the solubility of BCS II Class Itraconazole which is a low-bioavailable anti-cancer drug with limited dissolution rate, physico-chemically poorly soluble in water, low aqueous solubility of ~ 1 ng/ml at neutral pH and ~ 10 ng/ml at 0.1 N HCl by integrating with nanosponge using solid dispersion technique.
Nanosponges were obtained with crosslinked carbonate bonds of β –CD. As a result of this study, they found that the solubility of the drug increased by more than 27-fold in the presence of nanossponges and this ratio increased to 55-fold when copolyvidonum (PVP) was added as an auxiliary component in the nanosponge formulation. In addition to this finding, they showed that the dissolution profile of the drug was higher for the two formulations compared to the plain drug and that the crosslinked nanosponge formulation increased the bioavailability of itraconazole.
In another study, they investigated how the drug called Telmisartan (TEL) was affected by the NS obtained by using the solvent method of β –CD and DPC cross-linker at a molar ratio of 1: 4. They concluded that the solubility of telmisartan increased from 9.9 ug/ml to 45.92 ug/ml due to complexation with nanosponge [64]. In the same resarch of in vivo study, Cmax value of NS-TEL compared to pure TEL increased from 141 ng/ml to 258 ng/ml so that their bioavailability increased.
Shende et al. [51] investigated the effect of meloxicam on the solubility, stability and prolonge release with β–CD and NScontaining complexes. For this purpose, meloxicam drug was integrated with 1:1 molar ratio of beta cyclodextyrin and 1: 8 molar ratio of NS obtained with beta cyclodextrin and using pyromellitic dianhydride (PMDA) cross-linker.
They showed that the solubility of meloxicam which is 9.45 ug/ml in aqueous medium increased to 19.07 ug/ml by integration with beta-cyclodextrin alone. This value increased to 36.61 ug/ml in integration with NS, thus NS is a carrier that increased the solubility of the drug. In addition, the drug was released from NS in a controlled manner for 24 hours (release of pure drug during the first 2 hours was 45.23%, release in the formulation was 73.09% for 24 hours) and controlled release reduced the toxic side effect of the drug. They emphasized that NS loaded with a drug at a ratio of 1:1 w/w increased antiinflammatory and analgesic activity compared to pure meloxicam [51]. They also observed that there was no change in the release profile, particle size and stability in vitro by storing the drug at 60°C for 3 months.
Pushpalatha et al. [65] investigated the effects of NS, prepared using two different cross-linker s on Curcumin (CUR) which has a poor aqueous solubility of photoreactive and potent anticancer activity. For this purpose, different molar ratios of beta cyclodextrin with diphenyl carbonate (DFC) and pyromellitic dianhydride (PMDA) were applied by solvent method to obtain 1:2 optimum molar ratio of two different NS and then CUR was integrated into NSs with 1:1 molar ratio. Drug release, stability, solubility and cytotoxicity values of NS were compared with pure drug and with respect to each other. Accordingly, the solubility of PMDA crosslinked drug loaded NS (PMDA-CUR-NS) was 63.98%, while the solubility of DFC crosslinked drug loaded NS (DFC-CUR-NS) was 18.6%. in vitro drug release of PMDA-CUR-NS increased 16-fold compared to pure CUR and 5-fold compared to DFC-CUR-NS, whereas the in vitro cytotoxicity IC50 value of PMDA-CUR-NS decreased 2.2 times compared to pure drug and decreased 1.5 times compared DFC-CUR-NS. As a result, they emphasized that PMDA-NS is a more potential nanocarrier compared to DFCNS.
Frequent dosing is one of the disadvantages encountered in traditional drugs. In dosing using nanosonges, it is possible to protect the drug from environmental influences by providing a slow, sustained release of the drug over the entire dosage range by reducing the pharmacokinetic profile of the drug and reducing the frequency and side effects of the drug. A unique study was conducted by Cavalli et al. [66] for 3 model drugs by using NS.
Cavalli et al. [66] evaluated the effect of NS on their drug solubility drug release profiles by selecting 3 model drugs that exhibited both lipophilic (eg dexamethasone or flurbiprofen) and hydrophilic (eg doxorubicin) properties using beta cyclodextrin and DFC cross-linker . Studies have shown that the solubility of lipophilic drugs increases by about 15% by weight and 4% by weight for doxorubicin hydrochloride. As the drug release profile, doxorubicin is released very slowly at pH 1.1 in the physiological environment, whereas when released to pH 7.4 the release is faster. They also stressed that drug release from nanosponges was less than 10% for flurbiprofen and less than 20% for dexamethasone after 2 hours, confirming the strong interaction between the drug and nanosponge. As a result, Cyclodextrin based nanosponges showed that they carry lipophilic and hydrophilic drugs and have the ability to slowly release them to physiological environments, and they can be used to protect the lipophilic drugs from physiological environment such as stomach and increase their aqueous solubility.
A similar study showing the effects of NS on drug release and oral bioavailability of drugs in vitro was performed using low-solubility nifedipine drug [67]. As a result of the study, nifedipine was integrated into NS obtained by solvent method using beta cyclodextrin DFC cross-linker in a molar ratio of 1:4. Result showed that in vitro drug release profile increased by more than 78.4% in a controlled manner from drug loaded nanosponge as against of pure nifedipine, however, they showed that oral bioavailability increased 3.63 fold at Cmax versus pure drug.
NSs can be used as a drug delivery system to protect the encapsulated drugs from light, enzyme-induced, physical and chemical degradation. For this purpose, Anandam et al. [68] investigated the protective effect of nanosponges on photodegradation of quercetin by conducting separate irradiation tests for pure and complex quercetin at the same concentration, and as a result, it was noted that pure quercetin degradation was faster than that of quercetin encapsulated in nanospongens. In the same study, pure quercetin concentration showed more than 50% reduction in simulated intestinal fluid (SIF) over 6 hours, but no significant reduction was observed with NS encapsulated formulations, so NSs could be used effectively to protect drugs from the stomach. In addition, they emphasized that quercetine DPPH radical scavenging activity increased 569-fold with NS complex and its antioxidant activity increased significantly [68].
In another study, a drug called camptothecin (CPT), which inhibits the DNA topoisomerase enzyme and is particularly used in the treatment of prostate cancer [69] was investigated how NS protects camptothecin from degradation and increases in vitro anti-tumor activity. Minelli et al. [69] emphasized that the inclusion of CPT in β-Cyclodextrin nanosponge facilitates the cellular uptake of the drug and that the intracellular content of CPT increases over time up to 6 hours from treatment. This result confirmed that the introduction of CPT into the nanoparticle system prevents physiological deterioration that may occur in the extracellular environment and facilitates the accumulation of the drug into the cells.
Nanosponge is used passive targeting as a cosmetic agent for the skin as well as hydrogel and as a topical drug release system. Such a study was used by Sharma and Pathak [70] as an alternative carrier for targeting econazole nitrate (EN) to the skin using polymeric nanospongs with topical hydrogel formulations. The pharmacotechnical properties and irritation studies of EN loaded nanosponge hydrogels were tested on the mouse skin by selecting the best hydrogel that performs swelling, low stiffness, high viscosity, and controlled release for 12 hours. As a result, it has been emphasized by researchers that EN-loaded nanosponges may provide long-term drug release potential as well as some benefits such as frequency of administration, reduction in total dose, and reduction of dosedependent systemic side effects [70].
Investigating the topical application of hydrogel nanasponges, Gangadharappa et al. [71] integrated the lipophilic and low aqueous solubility celecoxib with prepared hydrogel nanasponge and studied pharmacokinetics and skin irritation on mice. It has been emphasized that optimized formulation NS-4 is non-irritating to mouse skin, so this dosage form can meet the requirements for human use, and that the resulting nanosponge hydrogels can be used as topical drug release for the use of celecoxib [71].
Pushpalatha et al. [72] prepared two different types of NS and investigated their effects on resveratrol, a weakly soluble, photosensitive drug. Accordingly, different molar ratios of beta-cyclodextrin with diphenyl carbonate and pyromellitic dianhydride cross-linker s were used to obtain NS-I and NS-II respectively, and then resveratrol integrated into NSs to synthesize RES-NS-I and RES-NS-II respectively. As a result, photodegradation, in vitro drug release, in vitro cytotoxicity and in vivo oral bioavailability studies were performed on mice.
Drug release rate from RES-NS-II in dissolution medium increased 2.5-3 times compared to RES-NS-I and photostability increased 2.3 times compared to RES-NS-I. However, it was emphasized that IC50 values of drug loaded NS showed a 1.5- fold decrease in cytotoxicity test compared to pure drug. As a result, RES-NS-II proved to be an effective nanocarrier for resveratrol compared to RES-NS-I [72].
In addition to the use of cyclodextrin-based NS as a drug delivery agent, it is used in many areas. For instance, NS could be used in protein delivery [73], in maintaining the catalytic competence, in the transmission and storage of gases such as oxygen, carbon dioxide [74], increasing stability of enzymes [75] and to carry substances such as peptides, antibodies and derivatives for biomedical applications that mentioned above. In addition, it is used in cosmetics [76] in agricultural [77] and in water purification porpuses [78]. In particular, in the transmission and storage of gases such as oxygen, carbon dioxide plays an important role in medical use for diagnostic or therapeutic purposes for biomedical applications. A lack of more oxygen supply can cause a variety of diseases, from inflammation to cancer. Therefore, it is sometimes complicated to deliver oxygen gas in a suitable means and dosage in clinical treatments. Cavalli et al. researched NSs structure for topical applications with regard to storage and slow release of oxygen gas over time [74]. Nanosponges can selectively absorb a measurable substance in an organism known biomarkers for the diagnosis purposes. Such study conducted by Longo et al. that nanosponge can gather rare cancer marker from blood [79]. From an environmental point of view, it could be a very effective agent to remove pollutants from water contaminated with persistent organic pollutants (POPs), such as chlorotoluenes, polychlorobiphenyls and chlorobenzenes or with heavy metals like lead, chromium, zinc, cadmium, etc.
Conclusion
Nanocarriers offer great advantages over conventional methods in view of drug targeting, drug delivery and drug release as well as other potentials. The main purpose of using these carriers can be summarized as increasing the saturation, biocompatibility and reducing the side effects of the molecules they carry, thus enabling the delivery and release of therapeutic agents to the target site. All these detailed researches show that Cyclodextrin based nanosponges are capable of encapsulating lipophilic as well as hydrophilic drugs, which can release drugs in a controlled and sustained manner in the target area. NSs is a promising nanocarrier with biocompatible, biologically usable properties that enhance the biocompatibility and aqueous solubility capacity of drugs by protecting them from the physiological environment and degradation.
NSs can be modulated into small particle size, spherical shape by controlling according to the cross-linking and betacyclodextrins molar ratio and by synthesis method that is used. Thus, they may increase the yield and specificity by retaining the encapsulated materials in the blood for a long time or using them with ligands for targeting.
Nanosongones have application in a wide variety of dosage forms such as tablets, capsules, aerosol, parenteral and topical, in medicine, pharmacy, as well as other important fields such as agriculture, cosmetics and the environment. As a result, NSs continue to be investigated as a promising nanocarrier system that can be used for the transport and deliver of active molecules to the target region with their multifunctional properties.
Apart from all these researches, there is much to be searched for and developed for NS in the future. For example, efforts to obtain functionalized NS and consequently increase drug loading capacity and drug release are limited except for a few publications. Obtaining functionalized NS or surface engineering studies of NSs may result in prolonged stay in the blood or increased yield and specificity for targeting sites using ligands. In addition, the delivering of NS to selected regions by using internal and external stimulants and investigation of drug release in cancer cells, modeling of NS integration into the cell can be considered as a future approach. However, in order to overcome the problems for nucleic acid delivery in gene delivery, positively charged NS synthesis studies should be conducted and NSs should be investigated as a fully effective nanocarrier system.
Acknowledgement
The authors thanks to Prof. Dr. Muberra Andac for important intellectual content and full support.
Conflict of Interest
Authors declare no personal or financial conflict of interest with any parties.
References
- Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA-Cancer J Clin 64: 9-29.
- Torchilin VP (2000) Drug targeting. Eur J Pharm Sci 11: S81-S91.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA-Cancer J Clin 68: 394-424.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2: 751-760.
- Jin SE, Jin HE, Hong SS (2014) Targeted delivery system of nanobiomaterials in anticancer therapy: From cells to clinics. Biomed Res Int 2014: 1-23.
- Webster DM, Sundaram P, Byrne ME (2013) Injectable nanomaterials for drug delivery: Carriers, targeting moieties, and therapeutics. Eur J Pharm Biopharm 84: 1-20.
- Subramanian MR, Selvamuthukumar S, Kilimozhi D (2017) Nanocarrier mediated combination drug delivery for chemotherapy–A review. J Drug Deliv Sci Technol 39: 362-371.
- Maeda H (2012) Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. P Jpn Acad B 88: 53-71.
- Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46: 6387-6392.
- Maeda H, Nakamura H, Fang J (2013) The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 65: 71-79.
- Haddish-Berhane N, Rickus JL, Haghighi K (2007) The role of multiscale computational approaches for rational design of conventional and nanoparticle oral drug delivery systems. Int J Nanomedicine 2: 315.
- Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, et al. (2010) Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release 141: 320-327.
- Jeong SY, Park SJ, Yoon SM, Jung J, Woo HN, et al. (2009) Systemic delivery and preclinical evaluation of Au nanoparticle containing β-lapachone for radiosensitization. J Control Release 139: 239-245.
- Chen YC, Liao LC, Lu PL, Lo CL, Tsai HC, et al. (2012) The accumulation of dual pH and temperature responsive micelles in tumors. Biomaterials 33: 4576-4588.
- Zhang J, Feng K, Cuddihy M, Kotov NA, Ma PX (2010) Spontaneous formation of temperature-responsive assemblies by molecular recognition of a β-cyclodextrin-containing block copolymer and poly (N-isopropylacrylamide). Soft Matter 6: 610-617.
- Ge J, Neofytou E, Cahill III TJ, Beygui RE, Zare RN (2011) Drug release from electric-field-responsive nanoparticles. ACS Nano 6: 227-233.
- Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A, et al. (2009) A novel magnetic crystal–lipid nanostructure for magnetically guided in vivo gene delivery. Nat. Nanotechnol 4: 598-606.
- Chen J, Guo Z, Wang HB, Gong M, Kong XK, et al. (2013) Multifunctional Fe3O4@ Ag hybrid nanoparticles as dual modal imaging probes and near-infrared light-responsive drug delivery platform. Biomaterials 34: 571-581.
- Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, et al. (2012) Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science 336: 604-608.
- Chakravarty P, Qian W, El-Sayed MA, Prausnitz MR (2010) Delivery of molecules into cells using carbon nanoparticles activated by femtosecond laser pulses. Nat Nanotechnol 5: 607-611.
- Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, et al. (2012) Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 337: 738-742.
- Lee Y, Miyata K, Oba M, Ishii T, Fukushima S, et al. (2008) Charge‐conversion ternary polyplex with endosome disruption moiety: A technique for efficient and safe gene delivery. Angew Chem Int Ed 47: 5163-5166.
- Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, et al. (2008) PEG-detachable polyplex micelles based on disulfide-linked block catiomers as bioresponsive nonviral gene vectors. J Am Chem Soc 130: 6001-6009.
- Broaders KE, Grandhe S, Fréchet JM (2010) A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J Am Chem Soc 133: 756-758.
- Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, et al. (2010) Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater 9: 923-928.
- Zhu L, Kate P, Torchilin VP (2012) Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano 6: 3491-3498.
- Jansook P, Ogawa N, Loftsson T (2018) Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int J Pharm 535: 272-284.
- Osmani RA, Kulkarni P, Manjunatha S, Vaghela R, Bhosale R (2018) Cyclodextrin nanosponge-based systems in drug delivery and nanotherapeutics: Current progress and future prospects. In: Organic materials as smart nanocarriers for drug delivery. (1st edn) p. 659-717. William Andrew Publishing.
- Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, et al. (2018) Cyclodextrins, from molecules to applications. Environ Chem Lett 16: 1361-1375.
- Davis ME, Brewster ME (2004) Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov 3: 1023-1025.
- Loftsson T, Jarho P, Masson M, Järvinen T (2005) Cyclodextrins in drug delivery. Expert Opin. Drug Deliv 2: 335-351.
- Frank DW, Gray JE, Weaver RN (1976) Cyclodextrin nephrosis in the rat. Am J Pathol 83: 367-382.
- Trotta F, Zanetti M, Cavalli R (2012) Cyclodextrin-based nanosponges as drug carriers. Beilstein J Org Chem 8: 2091-2099.
- Trotta F (2011) Cyclodextrin nanosponges and their applications. Cyclodextrins in pharmaceutics, cosmetics, and biomedicine: Current and future industrial applications.
- Swaminathan S, Cavalli R, Trotta F (2016) Cyclodextrin‐based nanosponges: A versatile platform for cancer nanotherapeutics development. Wıres Nanomed Nanobı 8: 579-601.
- Mognetti B, Barberis A, Marino S, Berta G, De Francia S, et al. (2012) In vitro enhancement of anticancer activity of paclitaxel by a Cremophor free cyclodextrin-based nanosponge formulation. J Incl Phenom Macrocycl Chem 74: 201-210.
- Swaminathan S, Pastero L, Serpe L, Trotta F, Vavia P, et al. (2010) Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur J Pharm Biopharm 74: 193-201.
- Rao M, Bajaj A, Khole I, Munjapara G, Trotta F (2013) In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J Incl Phenom Macro 77: 135-145.
- Sherje AP, Dravyakar BR, Kadam D, Jadhav M (2017) Cyclodextrin-based nanosponges: A critical review. Carbohydr Polym 173: 37-349.
- Ansari KA, Vavia PR, Trotta F, Cavalli R (2011) Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech 12: 279-286.
- Darandale SS, Vavia PR (2013) Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J Incl Phenom Macrocycl Chem 75: 315-322.
- Singireddy A, Subramanian S (2016) Cyclodextrin nanosponges to enhance the dissolution profile of quercetin by inclusion complex formation. Partıcul Sci Technol 34: 341-346.
- Dora CP, Trotta F, Kushwah V, Devasari N, Singh C, et al. (2016) Potential of erlotinib cyclodextrin nanosponge complex to enhance solubility, dissolution rate, in vitro cytotoxicity and oral bioavailability. Carbohydr Polym 137: 339-349.
- Trotta F, Cavalli R, Tumiatti W, Zerbinati O, Roggero C, et al. (2008) Ultrasound-assisted synthesis of cyclodextrin-based nanosponges. United States patent application EP Pat 1786841A1.
- Li D, Ma M (1998) Cyclodextrin polymer separation materials. Patent WO 9822197.
- Li D, Ma M (2000) Nanosponges for water purification. Clean Products and Processes 2: 112-116.
- Ma X, Chen Z, Chen R, Zheng X, Chen X, et al. (2011) Imprinted β‐cyclodextrin polymers using naringin as template. Polym Int 60: 1455-1460.
- Kyzas GZ, Lazaridis NK, Bikiaris DN (2013) Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohyd Polym 91: 198-208.
- Swaminathan S, Vavia PR, Trotta F, Cavalli R (2013) Nanosponges encapsulating dexamethasone for ocular delivery: formulation design, physicochemical characterization, safety and corneal permeability assessment. J Biomed Nanotechnol 9: 998-1007.
- Ferro M, Castiglione F, Punta C, Melone L, Panzeri W, et al. (2014) Anomalous diffusion of Ibuprofen in cyclodextrin nanosponge hydrogels: an HRMAS NMR study. Beilstein J Org Chem 10: 2715-2723.
- Shende PK, Gaud RS, Bakal R, Patil D (2015) Effect of inclusion complexation of meloxicam with β-cyclodextrin-and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surf B Biointerfaces 136: 105-110.
- Trotta F, Caldera F, Dianzani C, Argenziano M, Barrera G, et al. (2016) Glutathione bioresponsive cyclodextrin nanosponges. ChemPlus Chem 81: 439-443.
- Olteanu AA, Arama CC, Bleotu C, Lupuleasa D, Monciu CM (2015) Investigation of cyclodextrin based nanosponges complexes with angiotensin I converting enzyme inhibitors (Enalapril, captopril, cilazapril). Farmacia 63: 492–503.
- Pawar S, Shende P, Trotta F (2019) Diversity of β-cyclodextrin-based nanosponges for transformation of actives. Int J Pharm.
- Trotta F, Cavalli R, Tumiatti W, Zerbinati O, Roggero C, et al. (2008) Ultrasound-assisted synthesis of cyclodextrin-based nanosponges. United States patent application US 11/630,403.
- Osmani AM, Bhosale RR, Hani U, Vaghela R, Kulkarni P (2015) Cyclodextrin based nanosponges: Impending carters in drug delivery and nanotherapeutics. Curr Drug Ther 10: 3-19.
- Trotta F, Tumiatti V, Cavalli R, Rogero C, Mognetti B, et al. (2009) Cyclodextrin-based nanosponges as a vehicle for antitumoral drugs. WO 2009/003656:A1.
- Anandam S, Selvamuthukumar S (2014) Optimization of microwave-assisted synthesis of cyclodextrin nanosponges using response surface methodology. J Porous Mat 21: 1015-1023.
- Swaminathan S, Cavalli R, Trotta F (2016) Cyclodextrin‐based nanosponges: A versatile platform for cancer nanotherapeutics development. Wiley Interdiscip Rev Nanomed Nanobiotechnol 8: 579-601.
- Daga M, Ullio C, Argenziano M, Dianzani C, Cavalli R, et al. (2016) GSH-targeted nanosponges increase doxorubicin-induced toxicity “in vitro” and “in vivo” in cancer cells with high antioxidant defenses. Free Radic Biol Med 97: 24-37.
- https://www.observatorynano.eu/project/filesystem/files/HMN%20report. pdf
- Moya-Ortega MD, Alvarez-Lorenzo C, Concheiro A, Loftsson T (2012) Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int J Pharm 428: 152-163.
- Swaminathan S, Vavia PR, Trotta F, Torne S (2007) Formulation of betacyclodextrin based nanosponges of itraconazole. J Incl Phenom Macrocycl Chem 57: 89-94.
- Rao M, Bajaj A, Khole I, Munjapara G, Trotta F (2013) In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J Incl Phenom Macrocycl Chem 77: 135-145.
- Pushpalatha R, Selvamuthukumar S, Kilimozhi D (2018) Cross-linked, cyclodextrin-based nanosponges for curcumin delivery-physicochemical characterization, drug release, stability and cytotoxicity. J Drug Deliv Sci Technol 45: 45-53.
- Cavalli R, Trotta F, Tumiatti W (2006) Cyclodextrin-based nanosponges for drug delivery. J Incl Phenom Macro 56: 209-213.
- Cavalli R, Trotta F, Tumiatti W, Serpe L, Zara GP (2006) 5-Fluorouracile loaded beta-ctclodextrin nanosponges: in vitro characterisation and cytotoxicity. In: XIII International Cyclodextrin Symposium (p. 56). International Cyclodextrin Symposium. Turin, Italy.
- Anandam S, Selvamuthukumar S (2014) Fabrication of cyclodextrin nanosponges for quercetin delivery: physicochemical characterization, photostability and antioxidant effects. J Mater Sci 49: 8140-8153.
- Minelli R, Cavalli R, Ellis L, Pettazzoni P, Trotta F, et al. Nanosponge-encapsulated camptothecin exerts anti-tumor activity in human prostate cancer cells. Eur J Pharm Sci 47: 686-694.
- Sharma R, Pathak K (2011) Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm Dev Technol 16: 367-376.
- Gangadharappa HV, Prasad SM, Singh RP (2017) Formulation, in vitro and in vivo evaluation of celecoxib nanosponge hydrogels for topical application. J Drug Deliv Sci Technol 41: 488-501.
- Pushpalatha R, Selvamuthukumar S, Kilimozhi D (2018) Carbonyl and carboxylate crosslinked cyclodextrin as a nanocarrier for resveratrol: in silico, in vitro and in vivo evaluation. J Incl Phenom Macrocycl Chem 92: 261-272.
- Cavalli R, Ferruti P, Gerges I, Manfredi A, Ranucci E, et al. (2010) In vitro release modulation and conformational stabilization of a model protein using swellable polyamidoamine nanosponges of β-cyclodextrin. J Incl Phenom Macrocycl Chem 68: 183-191.
- Cavalli R, Ansari AK, Bisazza A, Giustetto P, Trotta F, et al. (2010) Nanosponge formulations as oxygen delivery systems. Int J Pharm 402: 254-257.
- Boscolo B, Trotta F, Ghibaudi E (2010) High catalytic performances of Pseudomonas fluorescens lipase adsorbed on a new type of cyclodextrin-based nanosponges. J Mol Catal B: Enzym 62: 155-161.
- Sapino S, Carlotti ME, Cavalli R, Ugazio E, Berlier G, et al. (2013) Photochemical and antioxidant properties of gamma-oryzanol in beta-cyclodextrin-based nanosponges. J Incl Phenom Macrocycl Chem 75: 69-76.
- Lien NR, Telford JR (2009) An investigation of the inclusion complex of cyclomaltoheptaose (β-cyclodextrin) with N-methylanthranilic acid in the solid state. Carbohydr Res 344: 2606-2608.
- Euvrard É, Morin-Crini N, Druart C, Bugnet J, Martel B, et al. (2016) Cross-linked cyclodextrin-based material for treatment of metals and organic substances present in industrial discharge waters. Beilstein J Org Chem 12: 1826-1838.
- Longo C, Gambara G, Espina V, Luchini A, Bishop B, et al. (2011) A novel biomarker harvesting nanotechnology identifies Bak as a candidate melanoma biomarker in serum. Exp Dermatol 20: 29-34.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences