Effect of Modification/Doping on Gas Sensing Properties of SnO2
S. Nagirnyak, T. Dontsova
DOI10.21767/2471-9838.100025
S. Nagirnyak and T. Dontsova*
Department of Chemistry, National Technical University of Ukraine “Igor Sikorsky KPI”, Kyiv 03056, Ukraine
- *Corresponding Author:
- Tetiana A. Dontsova
Department of Chemistry, National
Technical University of Ukraine
“Igor Sikorsky KPI”, Kyiv 03056, Ukraine
Tel: +38-093-753-66-36
E-mail: dontsova@ua.fm
Received Date: June 13, 2017 Accepted Date: July 03, 2017 Published Date: June 05, 2017
Citation: Nagirnyak S, Dontsova T. Effect of Modification/Doping on Gas Sensing Properties of SnO2. Nano Res Appl. 2017, 3:2.doi: 10.21767/2471-9838.100025
Abstract
Modification and doping are both terms which used to display improvement of sensory characteristics of materials, particularly their selectivity. But these processes are different in the way how they influence on material’s properties. This review concentrates on differences between modification and doping and their impact on parameters of sensitive materials for semiconductor gas sensors, in particular on characteristics of SnO2 as one of the most promising sensor material.
Keywords
Tin (IV) oxide; Additives; Modification; Doping; Selectivity
Introduction
Metal oxides SnO2, ZnO, In2O3, CdO are wide-bandgap n-type semiconductors and the most frequently used as a sensitive material for the gas sensors. They belong to a class of transparent conductive oxides due to a number of unique functional properties, of which the most important are the electrical conductivity, the visibility in a wide spectral range and the high reactivity of the surface [1-3].
Metal oxides based gas sensors are widely used due to their high sensitivity to harmful for human health or hazardous gases (such as CO, NO, NO2, H2, etc.) in conjunction with easy fabrication methods and low manufacturing costs. Tin (IV) oxide is the most promising sensor material among a wide set of semiconducting metal oxides [4-7].
In the technology of manufacturing sensors based on SnO2 the important place takes modification/doping of sensory material. This is due to the fact that pure SnO2, despite it’s obvious advantages (such as good surface adsorption properties, high chemical stability and mechanical strength, optical transparency in the visible region, good adhesion to glass and other surfaces, excellent electrical characteristic) [8,9] doesn’t provide sufficient selectivity for sensor device.
There are several approaches for improving the selectivity of gas sensor include choosing appropriate operating temperature depends on analyte, using additives and using sensor arrays [10]. Using additives can provide new active centers on the material’s surface or change electronic structure of material. In the first case, we are talking about modification of sensitive material, second variant belongs to the doping process.
There are a lot of papers devoted to study additives effect on selectivity of gas sensor devices [11-19]. However, there is a lack of attention on the difference between modification and doping processes. Therefore, the aim of this paper is to concentrate on differences and main principles of modification and doping processes.
Literature Review
Modification vs. Doping for selectivity improvement
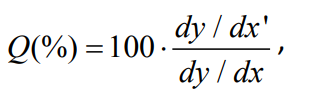
where y-an output signal or a change in the electrical resistance; x-given concentration of detected gas [21,22].
It is one of the most important parameters for gas sensor devices and creating of high-selective gas sensor is one of the most difficult tasks during device creation. One of the way for enhancing of sensor selectivity is introducing catalytically active additives in the sensing layer via doping or surface functionalization [23]. Additive can cause changing in the sensor performance depending on its doping concentration [11-14] ways of impregnating [24,25] sensor operating temperature [26,27] and the species of analyte gases [28].
Active components can be added in several different ways. Doping involves the addition of dopant to the prepared oxide. It can be due to the impregnation or mechanical mixing. Impregnation is related to ion-exchange/adsorption processes. It is the simplest method of preparing doped material. Solution containing the additive is contacted with powder of metal oxide and then the product is dried and/or heated at certain temperature. For example, to obtain gold/tin dioxide gas sensors Wang et al. dispersed SnO2 powders in HAuCl4 solution with further drying and calcinating procedures [29]. During the mixing process, the ready oxide and the compound of active component are mixed mechanically, after which the heat treatment is carried out to decompose the dopant compound. For instance, to obtain palladium-doped SnO2 gas sensors for selective detection of CO and CH4, SnO2 and PdCl2 powders were simply mixed in a mill, homogenized in a mixer and isostatically cold pressed. Then material was annealed in order to decompose the PdCl2 and to sinter the SnO2 crystallites [25]. In their work Choi and Oh synthesized La2O3-based SnO2 thick film gas sensors by ballmilling of commercial SnO2 and La2O3 powders in a ZrO2 jar with ZrO2 balls [30].
Modification is more complicated process. It comprises the addition of additives directly during the synthesis of the metal oxide. The so-called process of precipitation or coprecipitation. The undoubted advantage of this method is more uniform distribution of the active component. Precipitation is in principle a crystallization process and can occur in the bulk of the liquid. In almost all cases, the formation of a new solid phase in a liquid medium result from two elementary processes: formation of the smallest elementary particles of the new phase which are stable under the precipitation conditions; and agglomeration of the particles [31]. Gu et al. reported of obtaining Ni-doped SnO2 microstructures using wet synthesis technique from SnCl4·5H2O and NiCl2·6H2O solutions [32]. For the preparation of Pd-doped SnO2 nanofibers, the corresponding amount of PdCl2 was added to the solution [28]. Shan et al. synthesized SnO2- Fe2O3 interconnected nanotubes by mixing Fe(NO3)3·9H2O and SnCl2·2H2O solutions under magnetic stirring at room temperature and calcinating of resulting composite nanofibers [33].
One of subtypes of precipitation techniques is gelation route or the sol-gel method. This is a homogeneous process which results in a continuous transformation of a solution into a hydrated solid precursor (hydrogel). Sol-gel methods have been recognized for their versatility which allows control of the texture, composition, homogeneity, and structural properties of the finished solids [31]. Pd-doped SnO2-based sensor was obtained by Lim using sol-gel process. PdCI2 was added to the gel of SnO2 precipitates from the SnCI4 solution and mixture was calcined at 550°C in air [24]. To synthesize indium-doped SnO2 nanoparticles Kaur et al. prepared indium-doped sol by adding an appropriate amount of In2Cl3 in the tin oxide sol at its initial preparation stage [14].
Therefore, considering the processes of doping and modification from the point of view of synthesis technique, the difference between these two methods is in the ways and stages of the addition of active components. The process of doping is easier to implement. Although the modification allows to achieve a higher level of homogenization and homogeneity.
Additives can influence on material properties through two different mechanisms–chemical and electronic [20]. In chemical way, also known as catalytic, the reaction takes place at the material surface. Metal additive acts as catalyst from which compressed gas is transported to the surface of SnO2, where reacts with absorbed oxygen. And released electron leads to a decrease in resistance [34]. This scheme represents modification process (Figure 1a). A modification is a change or alteration to improve characteristics of material or device. In the case of semiconductor gas sensors modification of material’s microstructure means the creation of new active centers in relation of certain gases by using additives. The surface of nanostructures SnO2 is characterized by the presence of oxygen vacancies, which are active centers but non-selective because they allow to interact with different molecules from gas phase at the same time [35]. Thus, for increasing of selectivity of gas sensors based on tin (IV) oxide the chemical modification of surface is used. In this instance, the surface acquires new active centers of “receptor selectivity” which respond only to target gases.
The electronic mechanism introduces doping process. Here the reaction involves dopant atoms (Figure 1b) [20]. Term doping is used to describe the adding of “impurities” into material’s structure for improvement it’s chemical and physical properties. In the case of the electronic mechanism (so-called Fermi energy control mechanism), reducing gas reacts with the surface of the metal additive. As a result, electron released and transported to SnO2. Changes in the electron density near the surface of SnO2 lead to a decrease in resistance [34]. Additives can cause changing in the charge concentration of the SnO2 matrix, catalytic activities, the surface potential, formation of new donor or acceptor energy states and also influence on physical properties of material [20,36]. This in turn leads to changes in the electrical characteristics. Depending on impurities nature additives can result in increasing of conductivity because an extra electron is available in the lattice (donor impurities) or increasing of resistance because the effect of the oxygen vacancy donor levels is compensated by the acceptor levels (acceptor impurities) [37].
Thereby, the main difference between the electronic and catalytic mechanism is to transport particles between the additive and SnO2. In the electronic model takes place the transfer of electrons; in the chemical model-transportation of atoms. Figure 2 schematically shows both the detection mechanisms on metal oxide doped semiconductor.
But in principle, this division on two different mechanisms is quite conditional, because some additives have more broad impact and can influence on the electronic structure as well as create new adsorption sites [38].
Choose of additives for increasing selectivity of SnO2-based sensors
As dopants, most commonly used metals of platinum group-Pt, Pd, Ru, Rh [11,15,37,39], or oxide catalysts-Fe2O3 , La2O3, Cr2O3, V2O5 , NiO, CuO [16,17,30,33,40,41]. Important step-choice modifier for the gas and the change the reactivity of the material by changing the modifier concentration. The choice of dopant is carried out depending on the nature of the gas, clusters of noble metals used for doping sensor elements aimed at determining gas-oxidants (O2, NO2) and gases not have defined acid-base properties (CO, H2, CH4). For detection of basic and acidic gases using clusters of oxide catalysts-oxides of molybdenum and vanadium to identify the basic gases; oxides of copper, nikel, iron, lanthanum for detection of acid gases (Figure 3). However, some additives can increase selectivity and sensitivity to gases of different nature. For instance, indium-doped SnO2 thin films are sensitive to both reducing and oxidizing gases, depending upon the doping concentration and the operating temperature [14,42].
Discussion
Even at very small dopant concentrations a bulk and a surface effect are observed. The reason for these findings can be attributed to a shift of the Fermi level position due to the presence of additional donor levels in the band gap and to the appearance of surface acceptor levels [43]. That’s why usually, the additive loadings required for improved sensor performance are low (typically less than 10% mass or mole basis) [27]. For example, Choi et al. reported that selective detection of C2H5OH was observed with 0.08 wt% Pd SnO2 hollow nanofibers [28]. In their work Hübner et al. showed increasing of sensing parameters towards H2 and CO by using 0.2 wt% Pt: SnO2 sensor [38]. Wagn et al. examined the effect of Au loading of Au/SnO2 sensor for different CO concentrations. It was found that optimum Au loading was 2.86 wt%. Below this Au content the response to CO gas increased with the increase of the gold loading. But for Au loading more than 2.86 wt%. The response to CO gas decreased with the increase of the Au percentage [13]. In the case of PdOSnO 2 nanocomposites the maximum sensor response to CO was obtained at 0.1 mol% Pd. And the rising of Pd contents causes decreasing of sensor response [12]. SnO2-based sensors with good response to CO2 were synthesized by addition of 2.2 wt% of La2O3 [30].
Thus, taking into account, presented results of different researches, it can be concluded that not only nature of additives, but also their concentration has a huge impact on sensor properties.
Conclusion
In terms of data presented in contemporary scientific literature, selectivity of gas sensors can be improved by using additives as it allows to create new active sites as well as to impact on electrical properties. Both these changes lead to increasing of selectivity, but in different way. For choosing of additive the nature of detected gas should be considered. No less important step is finding of the optimum loading concentration for which the best sensor response can be achieved.
References
- Batzill M (2006) Surface science studies of gas sensing materials: SnO2. Sensors 6: 1345-1366.
- Wang C, Yin L, Zhang L, Xiang D, Gao R (2010) Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 10: 2088-2106.
- Nagirnyak S, Lutz V, Dontsova T, Astrelin I (2016) The effect of the synthesis conditions on morphology of tin (IV) oxide obtained by vapor transport method. Springer Proc Phys 183: 331-341.
- Pan J, Shen H, Mathur S (2012) One dimensional SnO2 nanosructures: Synthesis and application.1-12.
- Köck A, Tischner A, Maier T, Kast M, Edtmaier C, et al. (2009) Atmospheric pressure fabrication of SnO2-nanowires for highly sensitive CO and CH4 detection. Sensors and Actuators B: 160-167.
- Park JH, Lee JH (2009) Gas sensing characteristics of polycrastalline SnO2 nanowires prepared by polyol method. Sensors and Actuators B: 151-157.
- Qin L, Xu J, Dong X, Pan Q, Cheng Z, et. al. (2008) The template-free synthesis of square-shaped SnO2 nanowires: The temperature effect and acetone gas sensors. Nanotechnology 19: 1-8.
- Nagirnyak SV, Dontsova TA, Astrelin IM (2015) One-dimensional tin (IV) oxide nanostructures as gas-sensing materials. Research Bulletin of the National Technical University of Ukraine “Kyiv Politechnic Institute”. 5: 119-128.
- Dontsova TA, Nagirnyak SV, Zhorov VV, Yasiievych YV (2017) SnO2 nanosturctures: Effect of processing parameters on their structural and functional properties. Nanoscale Res Lett 12: 1-7.
- Nagirnyak SV, Dontsova TA (2015) Ways for improvement selectivity of semiconductor gas sensors. Young scientist 10: 15-17.
- Ramgir NS, Hwang YK, Jhung SH, Mulla IS, Chang JS (2006) Effect of Pt concentration on the physicochemical properties and CO sensing activity of mesostructured SnO2. Sensors and Actuators B 114: 275-282.
- Yuasa M, Masaki T, Kida T, Shimanoe K, Yamazoe N (2009) Nano-sized PdO loaded SnO2 nanoparticles be reverse micelle method for highly sensitive CO gas sensor. Sensors and Actuators 136: 99-104.
- Wang S, Zhao Y, Huang J, Wang Y, Kong F, et al. (2006) Preparation and CO gas-sensing behavior of Au-doped SnO2 sensors. Vacuum 81: 397-397.
- Kaur J, Kumar R, Bhatnagar MC (2007) Effect of indium-doped SnO2 nanoparticles on NO2 gas sensing properties. Sensors and Actuators B 126: 478-484.
- Aguilar LJ, Maldonado A, Olvera M (2006) Gas-sensing characteristics of ruthenium-doped SnO2 thin films in a propane atmosphere. Sensors and Actuators B Chemical: 1-4.
- Liu L, Zhang T, Wang L, Li S (2009) Improved ethanol sensing properties of Cu-doped SnO2 nanofibers. Materials Letters 63: 2041-2043.
- Yang H, Jin W, Wang L (2003) Synthesis and characterization of V2O5-doped SnO2 nanocrystallites for oxygen-sensing properties. Materials Letters 57: 3686-3689.
- Nakatani Y, Matsuoka M (1982) Effects of sulfate ion on gas sensitive properties of α-Fe2O3 ceramics. Jpn J Appl Phys 21: L758
- Epifani M, Arbiol J, Pellicer E, Comini E, Siciliano P, et. al. (2008) Synthesis and gas-sensing properties of Pd-doped SnO2 nanocrystals. A case study of a general methodology for doping metal oxide nanocrystals. Cryst Growth Des 8: 1774-1778.
- Bochenlov VE, Sergeev GB (2010) Sensitivity, selectivity and stability of gas-sensitive metal-oxide nanostructures. Metal oxide nanostructures and their applications V.3: 31-52.
- Ho GW (2011) Gas sensor with nanostructured oxide semiconductor materials. Sci Adv Mater 3: 150-168.
- Franke ME, Koplin TJ, Simon U (2006) Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2: 36-50.
- Smulko J, Trawka M, Granqvist CG, Ionescu R, Annanouch FE, et al. (2014) New approaches for improving selectivity and sensitivity of resistive gas sensors: A review. Proc of the 8th Intern Conf of Sensing Techn, Liverpool, UK. pp. 13-18.
- Lim CB, Oh S (1996) Microstructure evolution and gas sensitivities of Pd-doped SnO2-based sensor prepared by three different catalyst-addition processes. Sensors and Actuators 30: 223-231.
- Tournier G, Pijolat C, Lalauze R, Patissier B (1995) Selective detection of CO and CH4 with gas sensors using SnO2 doped with palladium. Sensors and Actuators 27: 24-28.
- Liu L, Zhang T, Li S, Wang L, Tian Y (2009) Preparation, characterization, and gas-sensing properties of Pd-doped In2O3 nanofibers. Materials Letters 63: 1975-1977.
- Bârsan N, Tomescu A (1995) The temperature dependence of the response of SnO2-based gas sensing layers to O2, CH4 and CO. Sensors and Actuators 26: 45-48.
- Choi JK, Hwang IS, Kim SJ, Park JS, Park SS (2010) Design of selective gas sensors using electrospun Pd-doped SnO2 hollow nanofibers. Sensors and Actuators 150: 191-199.
- Wang S, Zhao Y, Huang J, Wang Y, Wu S, et al. (2006) Low-temperature carbon monooxide gas sensors based gold/tin dioxide. Solid-State Electronics 50: 1728-1731.
- Choi JY, Oh TS (2013) CO sensitivity of La2O3-doped SnO2 thick film gas sensor. Thin Sold Films 547: 230-234.
- Schwarz JA (1995) Methods for preparation of catalytic materials. Chem Rev 95: 477-510.
- Gu C, Guan W, Liu X, Gao L, Wang L, et al. (2017) Controlled synthesis of porous Ni-doped SnO2 microstructures and their enhanced gas sensing properties. J Alloy Compd 692: 855-864.
- Shan H, Liu C, Liu L, Zhang J, Li H (2013) Excellent toluene sensing properties of SnO2−Fe2O3 interconnected nanotubes. Appl Mater Interfaces 5: 6376-6380.
- Miller TA, Bakrania SD, Perez C, Wooldridge MS (2006) Nanosturctured tin dioxide materials for gas sensor applications. Functional Nanomaterials 30: 453-476.
- Krivetsky VV, Rumyantseva MH, Gaskov AM (2013) Chemical modification of nanocrystalline tin dioxide for selective gas sensors. Adv Chem 82: 917-941.
- Ali SM, Hussain ST, Bakar SA, Muhammad J, Rehman N (2013) Effect of doping on the structural and optical properties of SnO2 thin films fabricated by aerosol assisted chemical vapor deposition. Journal of Physics: Cinference series 439: 1-10.
- Hübner M, Bârsan N, Weimar U (2012) Influences of Al, Pd and Pt additives on the conduction mechanism as well as the surface and bulk properties of SnO2 based polycrystalline thick film gas sensors. Sensors and Actuators B Chemical 171: 172-180.
- Hübner M, Koziej D, Bauer M, Bârsan N, Kvashnina K, et al. (2011)The structure and behavior of platinum in SnO2-based sensors under working conditions. Angew Chem Int Ed 50: 2841-2844.
- Cho YH, Liang X, Kang YC, Lee JH (2015) Ultrasensitive detection of trimethylamine using Rh-doped SnO2 hollow spheres prepared by ultrasonic spray pyrolysis. Sensors and Actuators 207: 330-337.
- Gu C, Cui Y, Wang L, Sheng E, Shim JJ (2017) Synthesis of the porous NiO/SnO2 microspheres and microcubes and their enhanced formaldehyde gas sensing performance. Sensors and Actuators B 241: 298-307.
- Kasar RR, Gosavi SR, Chosh A, Deshpande NG, Sharma RP (2015) Influence of Cr doping on structural, morphological and optical properties of SnO2 thin film prepared by spray pyrolysis technique. IOSR Journal of Applied Physics 7: 21-26.
- Gaskov AM Nanocrystalline semiconductor materials for chemical and physical sensors. Faculty of Chemistry, Moscow State University. www.lssm.inorg.chem.msu.ru. [In Russian]
- Rebholz J, Jaeschke C, Hübner M, Pham D, Mädler L, et al. (2012) Conduction Mechanism in undoped and antimony doped SnO2 based FSP gas sensors. The 14th International Meeting on Chemical Sensors. pp. 105-108.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences



