Functional Coatings by Incorporating Nanoparticles
Swati Gaur and AS Khanna
Swati Gaur and AS Khanna*
Department of Metallurgical Engineering and Materials Science, IIT Bombay, Powai, India
- *Corresponding Author:
- AS Khanna
Department of Metallurgical Engineering and Materials Science, IIT Bombay, Powai, India
Tel: 09967671883
E-mail: khanna@iitb.ac.in
Received date: October 27, 2015, Accepted date: November 12, 2015, Published date: November 16, 2015
Citation: Gaur S, Khanna AS. Functional Coatings by Incorporating Nanoparticles. Nano Res Appl. 2015, 1:1.
Abstract
Use of nanoparticle addition in coatings has been described to formulate selfhealing and antifouling coatings. The first step in both these cases was to create a hydrophobic surface for which a highly reactive fluoro silane was used. The second property in each case was achieved by the addition of nano-particles. In self-cleaning coatings, easy sliding of water droplet was achieved by the addition of nano-ZnO and nano-silica, while, in the case of anti-fouling coating, this was achieved by addition nano silica, which provided a nano-roughness surface, which was so smooth to avoid sticking any barnacles or seaweeds.
Introduction
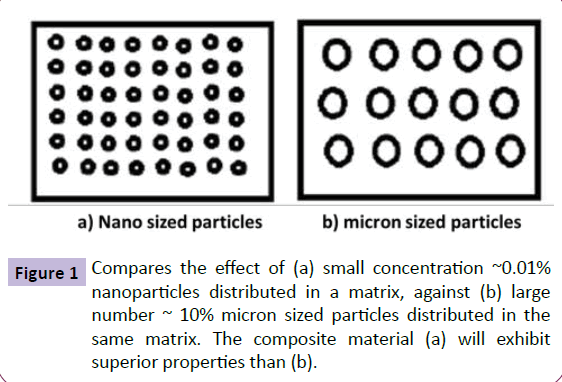
"Nanotechnology" is science to describe effects, which arise from the quantum structure of fine particles. Nanoparticles are generally considered atoms or molecules with at least one dimension of less than 100 nm. The interesting properties of nanoparticles are due to the high surface area to volume ratio. The extremely small size of nanoparticles offer high surface to volume ratio and provide the desired functionality when present in a very small concentration, compared to the bulk counterpart (Figure 1). It has well been proven that materials with high surface areas have enhanced physical, chemical, mechanical, optical or magnetic properties.
Because of this effect, nano additives in paint coatings are showing superior performance. There are several examples of addition of nanoparticles such as nano-ZnO, nano-alumina, nano silica etc. which have helped in enhancing properties such as corrosion resistance, mechanical properties and UV blocking effect [1]. Many techniques such as the the sol-gel method of synthesizing coatings results in nano-structured coatings which have much superior properties than the conventional organic coatings [2-5].
Functional coatings are defined as those, which perform efficiently a specific property of the substrate, in addition to its conventional properties. Classical examples of functional coatings are self-cleaning, anti-graffiti, antifouling, hydrophobic, self-healing etc. Silanes based sol–gel coatings are the subject of extensive research these days [6]. Such coatings have superior properties than conventional organic coatings and are in the category of "green coatings" as they do not use any toxic solvent, heat or any toxic raw material [7]. The sol-gel coating technology is very versatile, can be applied on many metals, and is compatible with several paint systems. They have the advantage of being environmentally friendly with ease of application on the metal surface. They can be tailored easily with the incorporation of nanoparticles to achieve the desirable properties for their application in different areas.
Various paint industries are considering the use of nanomaterials, such as nano-silver, photo-catalytically active nano-titanium dioxide or nano-silica dioxide to improve paint properties, such as water repellency, scratch resistance and antimicrobial properties. Polyurethane coatings containing alumina and silica nanoparticles can be used to improve the scratch resistance of clear coat polyurethane coatings [8]. Incorporation of silver nanoparticles in paints act as a depot of silver ions to impart antimicrobial properties. Particle size and surface coating tune the release of the ions. These silver ions penetrate into a bacterial cell, interact with thiol groups of vital enzymes, and deactivate them, which leads to dysfunction and death of cells [9,10]. The lacquer and paint industry also uses photo-catalytically active nano-titanium dioxide as biocide [11-13]. Nano-silica dioxide is widely used in paints to improve scratch resistance, water repellence, and helps in protecting the paint against corrosion and provides the products with high gloss [14]. Nanoparticle additives are also attractive materials for corrosion protection because of their high surface areas that allow them to function as carriers for molecular corrosion inhibitors [15].
Mechanism
The size, shape and chemical properties of the nanoparticles (i.e., solubility parameters, additional reactive groups) produce different results. The surface density plays an important role for dispersion in organic materials. The dispersion and solubility of nanoparticles can be smartly adjusted by the choice of surface groups. The presence of additional reactive groups allows the nanoparticles to be crosslinked to provide increased stability or durability to the cured coating [15].
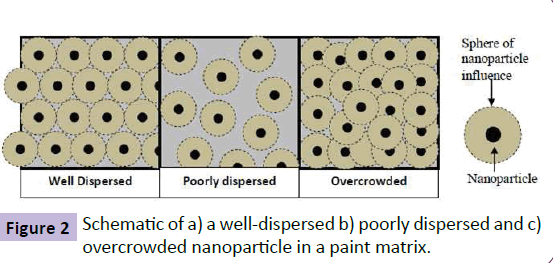
The mechanisms to explain the functioning of nano-additives/ nano-particles based on the increase in the surface area is known as the Continuum Theory. It has been observed that nanoparticles in coating can act effectively and provide coatings with the uniform property even when the particles are not attached to each other. From the various works carried out on nano-particles, it has been observed that optimum effect of nanoparticle addition is observed at a particular concentration of nanoparticles with a uniform distribution. As per the continuum theory of distribution of nanoparticles, effective dispersion and distribution of nanoparticles are the key criteria for the performance of nano-particles in any medium or matrix. Agglomeration of nano-particles is undesirable as it makes nanoparticles lose their high surface area and thus the desired functional properties [1,16-18]. This is best illustrated in Figure 2, 2a is a well-dispersed nanoparticle and maintains its influence throughout the coating whereas Figure 2b depicts less concentration and poorly dispersed nanoparticles with their ‘sphere of influence’ not carried over to the immediate next particle. Figure 2c, however, shows excess particles, which lose their influence due to overcrowding and overlapping with each other. Thus, optimum concentration and uniform distribution is one of the requirements to get the maximum positive effect of nanoparticle addition in paint coatings.
Use of Nanoparticles in Coating Applications
Let us now give two examples where nanoparticle addition has resulted in creating functional coatings:
1) Self-cleaning applications
For various applications such as self-cleaning of solar panels, glass windows, stain resistant textiles, corrosion prevention and anti-biofouling surfaces, creating a hydrophobic surface is the first step [1]. In order to create a hydrophobic surface, two requirements must be met. First, the surface should have low surface energy (hydrophobic). Second, the surface should have a roughness in nano-range. Such a surface can be achieved by a combination low sliding angle and high contact angle, for example, the water contact angle on a lotus leaf is as high as 160° with a rolling angle of about 2°, which is considered as a high performance superhydrophobic self-cleaning surface. The high contact angle can be attained by lowering the surface energy. The commonly used reactive molecules for low surface energy modification are mainly long alkyl chain thiols, alkyl or fluorinated organic silanes, per-fluorinated alkyl agents, long alkyl chain fatty acids, polydimethylsiloxane-based polymers or other polymers, or their combinations [11] and low sliding angle can be achieved by creating a surface where the contact of the bubble with the surface can be minimized, which can be achieved by several methods. The most appropriate method is by modifying the surface by incorporation of nano-particles and by creating micro or nanostructured features, which can reduce the contact area between the surface and the water droplet. The water droplets hence do not wet the surface and easily slide to remove dirt [2]. Moreover, surface roughness can be enhanced by introducing nanoparticles such as nano-ZrO2, nano-ZnO and nano-TiO2, nano- SiO2. The superhydrophobic surface created by sol−gel methods yields needle-like, surface roughness because of aggregation of nanoparticle clusters, this minimizes contact area, enhancing non-wetting behaviour. Unfortunately, the superhydrophobic nature of these kinds of surfaces is vulnerable to mechanical damage deteriorating surface roughness. Nakajima et al. [19] established crater like roughness as a more robust alternative. Phase separation and subsequent thermal decomposition of an organic polymer dispersion from within silica matrix yielded thin films with voids akin to craters. Further, post-functionalization with fluoro silanes, these surfaces yielded contact angles above 150° and pencil hardness above 4H. But this kind of approaches has been relatively under-explored in literature till now, with only a few examples utilizing crater roughness [20].
GPTMS-MTMS-HMMM based neat sol-gel coating was deposited on an aluminium surface which exhibited a contact angle of about 70-75° with RMS value of 3 nm. The neat sol-gel was hydrophobically modified with a six carbon atom water based fluoro-polymer emulsion (FE) used as the hydrophobic coprecursor to achieve enhanced hydrophobic and water repellent properties. The 30 wt% FE modified sol-gel system resulted in maximum improvement in hydrophobicity out of all with a contact angle of 118° and sliding angle of 70°. The surface roughness achieved in this case was about 37 nm.
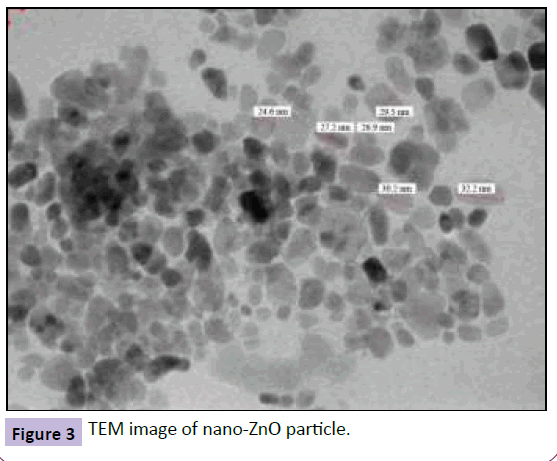
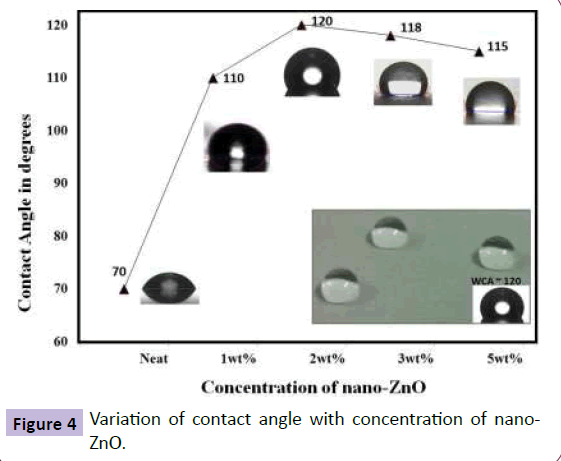
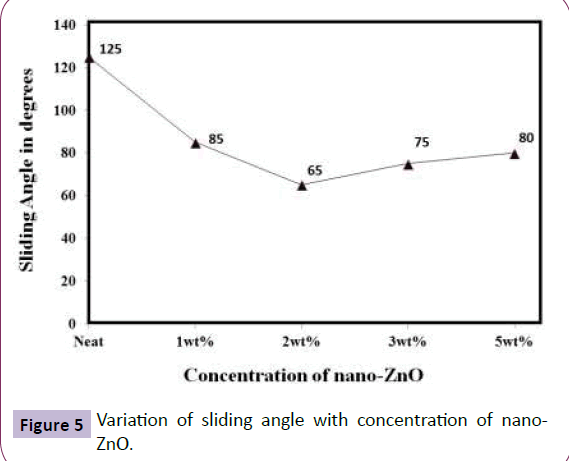
Further, incorporation of nano-ZnO (Figure 3 shows the TEM image of the nano-ZnO) in FE modified sol-gel resulted in surface roughness of 105 nm and this resulted in a marginal increase in hydrophobicity due to hydrophilic nature of nano-ZnO particles. The contact angle achieved was 120° with sliding angle of 65° (Figure 4 and 5).
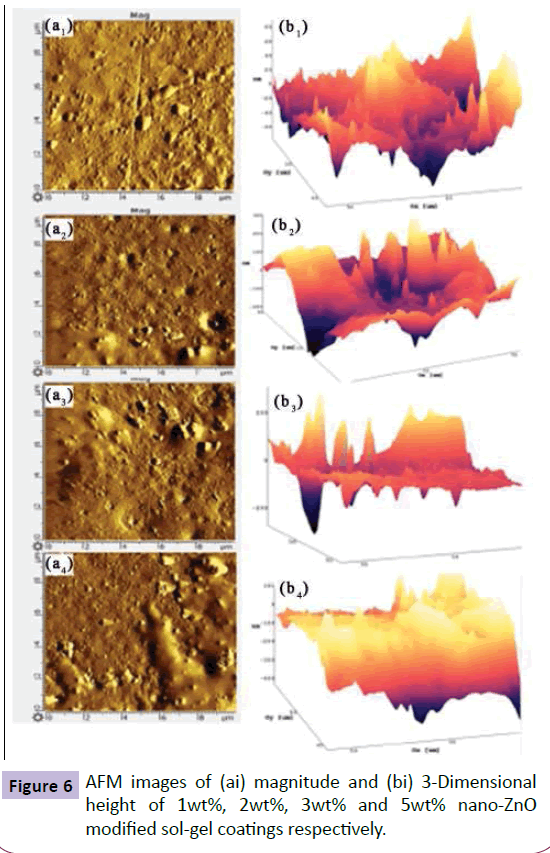
Figure 6 (ai) highlighted the nanoparticle distribution pattern. The magnitude of nano-clusters increases with increase in nano-ZnO content with uniform and dense distribution of nano-particles at particles at 2 wt% concentration. Hence, enhanced surface roughness of 105 nm was achieved after nano-ZnO modification resulting in higher contact angle of 120° and SA of 65º.
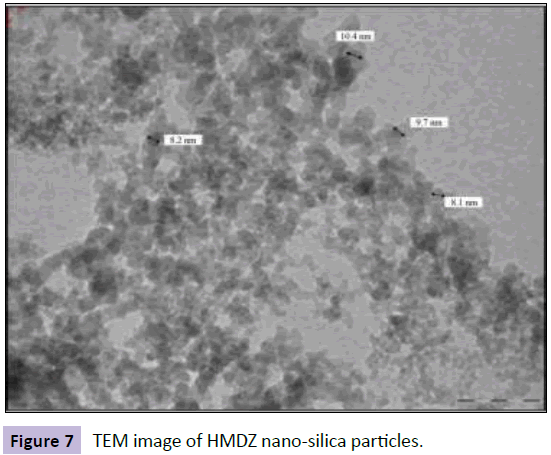
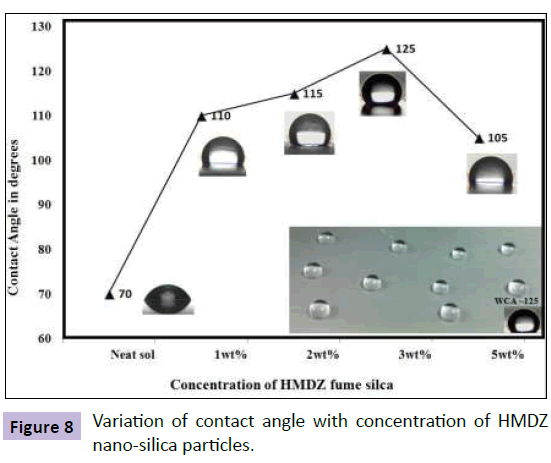
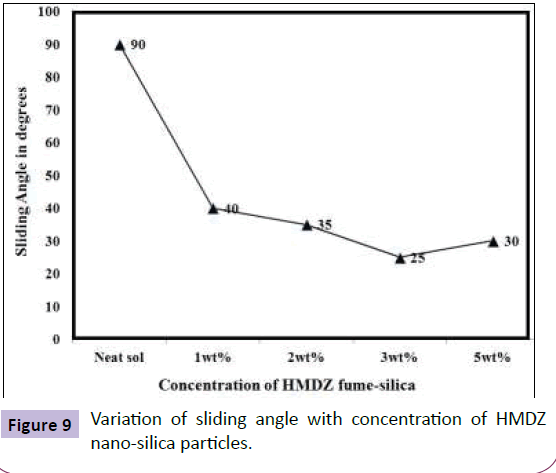
A further modification was achieved by using various functionally modified nano-silica particles out of which hexamethyldisilazane (HMDZ) nano-silica particles(Figure 7 shows the TEM of the HMDZ modified nano silica particles) resulted in maximum surface roughness of 95 nm with a contact angle of 125° and sliding angle of 25°(Figures 8 and 9). HMDZ-FE sol-gel coatings when applied on non-metallic substrates viz. cotton, wood, paper, concrete and cardboard, resulted in superhydrophobicity with contact angle >135° and sliding angle <5°[21,22].
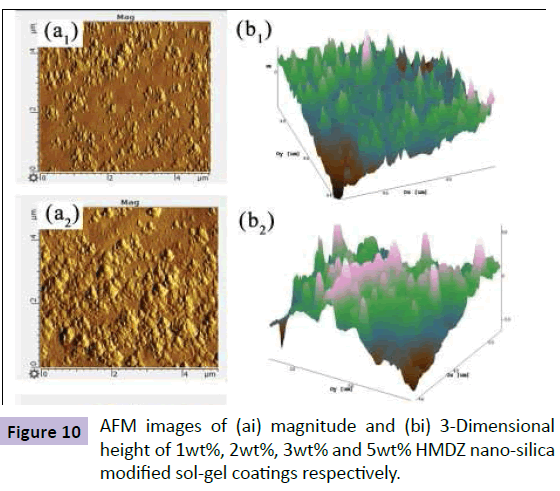
From the AFM images (Figure 10), it can be seen that magnitude of nano-clusters increases with an increase in HMDZ nano-silica content with uniform and dense distribution of fume silica particles at 3 wt% concentration. The smaller protrusions rest on the top layers, whereas the larger aggregations form the bottom layers. Thus, the multi-scaled roughness is created by the aggregations of nanoparticles resulting in protruding nubs, covering the whole surface. This assures, in fact, that the surface contact area available to water droplet was very low, while the hydrophobic nanoparticles prevented penetration of water into the valleys resulting in excellent sliding behaviour of water droplets with a contact angle of 125° and SA of 25° [23].
2) Antifouling coating applications
After achieving desirable results and success in self-cleaning superhydrophobic coatings, the same was applied on conventional epoxy based coatings. Environment-friendly antifouling coatings were developed, to prevent the settling of marine organisms on artificial immersed structures such as the hull of a ship. The use of traditional antifouling coatings like tributyltin (TBT) has been banned since 2008 due to environmental concerns. Hence, it was desired that the coating primarily was environment-friendly while possessing properties such as hydrophobicity, ultra smoothness (roughness only in angstrom level) and mechanical strength to sustain physical damage due to day-to-day activities.
Foul release property was achieved by converting a hydrophilic epoxy surface into a hydrophobic one by modifying the resin. This was done by incorporation of two types of surface energy showed excellent hydrophobicity with contact angle ranging from 98.3° to 121° compared to 74.85° for neat epoxy. Elemental and chemical analysis revealed that the silicone and fluoro chains have migrated to the surface due to their low surface energy and synergistically contributed in releasing the foulants. Even though both Nano additives reduce the surface energy of the coatings, their contribution to elastic modulus was different. Silicone-based polymers reduce the elastic modulus of the coating by plasticizing it. This results in a very soft coating, which was prone to physical damage, which is an undesirable feature, especially in the case of icebreakers, landing crafts, tugs, etc. where the hull is subjected to physical abuse. On the other hand, fluoro polymers stiffen the coating due to an immobile and hard back bone. This feature is also related to bio-adhesive-coating interface failure mechanism in both cases. Therefore, a foul release coating with both types of nano additives will not only have low surface energy but also have better mechanical properties than ‘silicone-only’ foul release coating, due to the synergistic effect of both fluoro and silicone chains. Foul release property was further enhanced by creating an ultra smooth surface so that, it inhibits the organism to settle by denying it to anchor. This high level of ultra smoothness was achieved with the help of both the nano-additives, which are excellent levelling agents. The shallow water immersion tests carried out in harbour showed considerable improvement in foul release behaviour of the modified coating with respect to neat epoxy.
In order to prove the anti-fouling effect, the panels were sandblasted to SSPC SP10 (SA 2 ½) standard. These were immediately coated with primer supplied by Naval Dockyard, Mumbai. The panels were then coated with one coat of epoxy based anticorrosive coating. After allowing recommended coating intervals as per product data sheets, one coat of antifouling coating (prepared in the lab) was applied as a top coat. All coatings were applied on the panels using a brush. No tie coat was used between anticorrosive and top coat. The total DFT of the paint system was measured and found to be in the range 450 ± 10 μ. These panels were then subjected to sea water immersion testing as per ASTM D 3623 at Naval Dockyard, Mumbai premises. A total of five panels were prepared per batch (Table 1).
| Panel | Name | Paint Scheme | |
|---|---|---|---|
| 1 | No top coat | Primer | 50 m |
| Anticorrosive paint | 150 m | ||
| Top coat | -- | ||
| 2 | Sample control |
Primer | 50 m |
| Anticorrosive paint | 150 m | ||
| Top coat | Neat Epoxy | ||
| 3 | Sample A | Primer | 50 m |
| Anticorrosive paint | 150 m | ||
| Top coat | 150 m Antifouling paint | ||
Table 1 Paint Scheme of each panel.
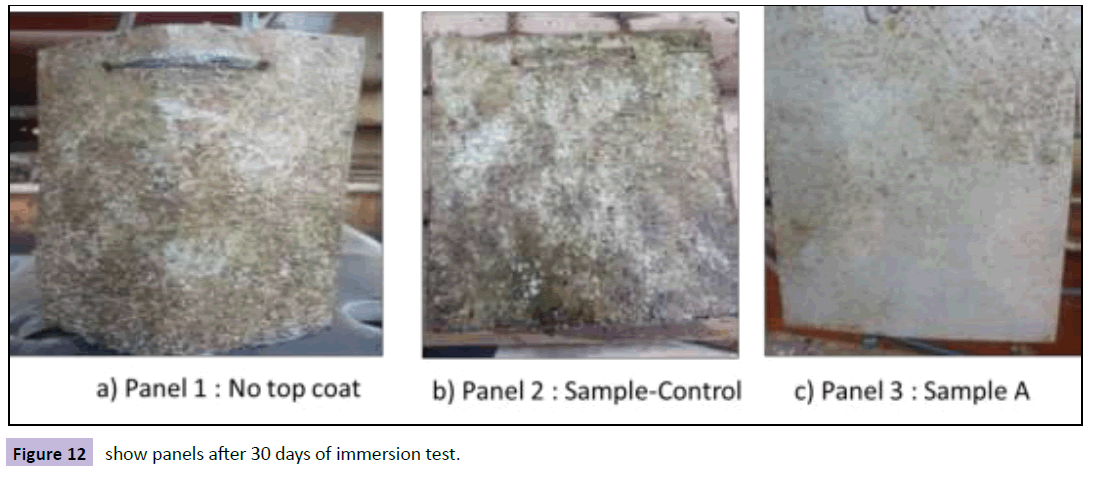
The panels were taken out of the water after 30 and 60 days of immersion and visually inspected for the amount of fouling. Photographs of panels were taken immediately after taking out of the water. This is to keep a record of the amount of fouling accumulated on the panel so that a comparative analysis can be undertaken. The extent of fouling per panel is a function of the area of coverage as well as the type of fouling.
The panels which were immersed for 30 days (Figure 12) showed organisms like bryozoans and hydroids along with small barnacle growth. After 30 days immersion, sample-A emerged as the least fouled coating. Both control panels had excessive fouling as compared to other panels.
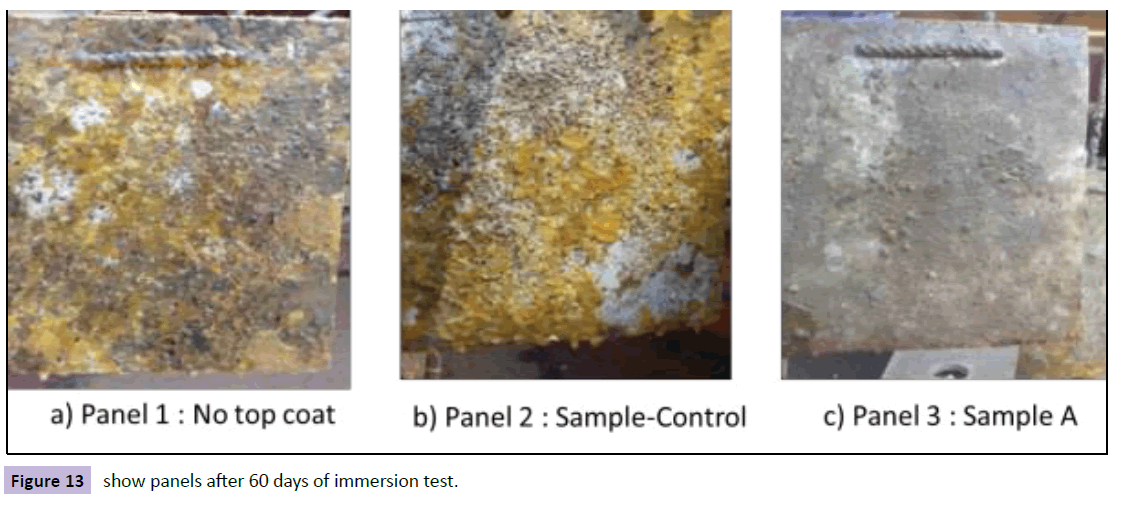
The panels, which were immersed for 60 days, showed further growth of fouling. There was the presence of barnacles as well. After 60 days immersion, panel A again emerged as the least fouled coating as shown in Figure 13.
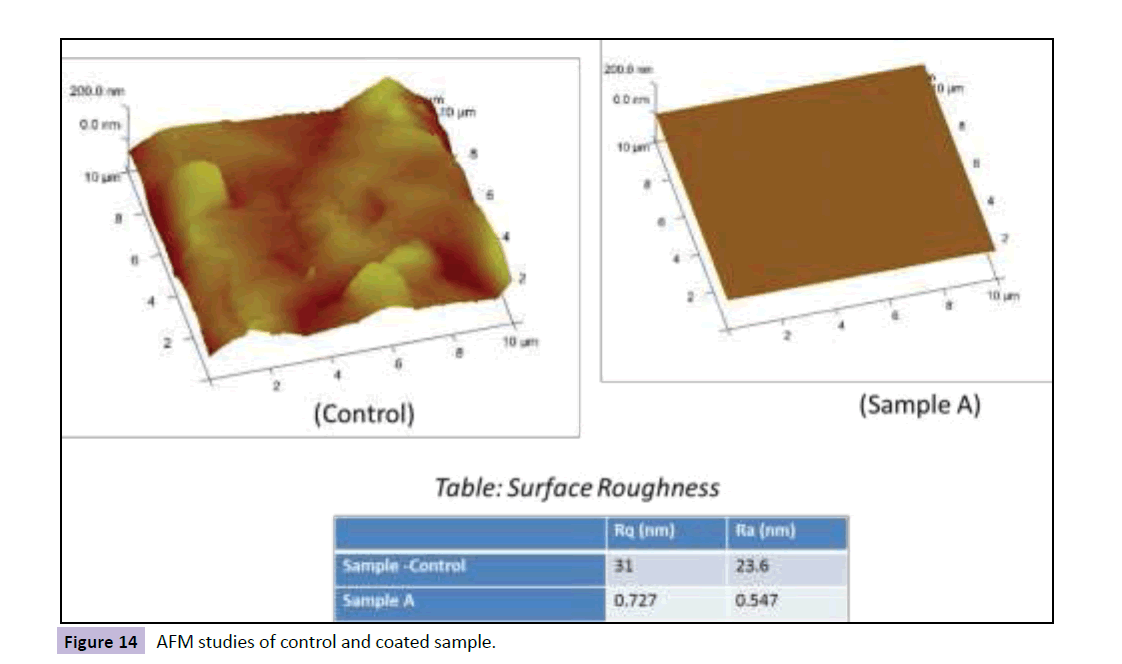
The foul release effect is achieved by two parts. One of them was by increasing hydrophobicity and other by achieving an ultra smooth surface. In other words, the absence of roughness up to nano level is required. In order to ascertain whether this physical condition is achieved, AFM analysis was carried out. The images obtained from AFM studies and representative roughness values of surfaces obtained for control and coated surface. It was observed that Sample A roughness much lesser as compared to control sample. Thus, sample A can be classified as the ultra-smooth surface. An ultra-smooth surface inhibits the organism to settle. In other words, even if fouling takes place, if the adhesion strength between fouling and surface is minimized, the fouling can be easily removed with small stresses. The stress required to remove the fouling is so small that, the fouling peels off even when the ship is underway in its operational speed. The surface morphology, in this case, is ultra-smooth and complete absence of roughness up to nano level denies the bio-adhesive to anchor on to the surface and prevents mechanical interlocking of bioadhesive and substrate. This level of ultra smoothness is achieved with the help of silicone and fluoropolymer additives, which are excellent levelling agents Figure 14 [24].
Conclusion
Nanotechnology is playing an important role in modifying several existing conventional technologies. Lower size with large surface area is one of the important factors to cause this improvement. Optimum concentration and its uniform distribution are other essential factors. An example of Self-cleaning coating demonstrated how the presence of nanoparticle helps in creating surface roughness thereby helping to reduce the contact of a water drop with the surface, thereby making it slide smoothly at a very small angle. The highest contact angle of about 125° was obtained with HMDZ modification, which resulted in improved water repellent properties, corrosion resistance and mechanical properties. Such behaviour is related to both the rough morphology and the intrinsic low surface energy of the HMDZ-silica particles that stem from the chemical nature of the tri-methylated groups grafted onto the particle surface. These tri-methyl groups give rise to the asymmetric molecular forces responsible for water repellent properties. In the same way, the anti-fouling effect was achieved by the addition of nano-silica particles, which are excellent levelling agents that help in making the surface so smooth that, made barnacles and sea-weeds difficult to stick on the ship hull. This was very well proved by the exposure of panels in seawater for 60 days.
References
- Dhoke SK, BhandariR, KhannaAS (2009) Effect of nano-ZnO addition on the silicone-modified alkyd-based waterborne coatings on its mechanical and heat-resistance properties. Progress in Organic Coatings 64: 39-46.
- Pathak SS,KhannaAS (2008) Synthesis and performance evaluation of environmentally compliant epoxysilane coatings for aluminum alloy. Progress in Organic Coatings 62: 409-416.
- Pathak SS,KhannaAS (2009) Investigation of anti-corrosion behavior of waterborne organosilane–polyester coatings for AA6011 aluminum alloy. Progress in Organic Coatings 65: 288-294.
- Pathak SS, KhannaAS, SinhaTJM (2007) HMMM cured corrosion resistance waterborne ormosil coating for aluminum alloy. Progress in Organic Coatings 60: 211-218.
- Raymond HF (2009)Nanocomposite and Nanostructured Coatings: Recent Advancements, in Nanotechnology Applications in Coatings.American Chemical Society pp. 2-21.
- vanOoij WJ, et al. (2005) Corrosion Protection Properties of OrganofunctionalSilanes—An Overview. Tsinghua Science & Technology 10: 639-664.
- vanOoij, WJ, et al. (2005) Corrosion Protection Properties of OrganofunctionalSilanes—An Overview. Tsinghua Science & Technology 10: 639-664.
- Matthew FC, et al. (2009) Effects of Alumina and Silica Nanoparticles on Polyurethane Clear Coating Properties, in Nanotechnology Applications in Coatings.American Chemical Society pp. 210-231.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, et al. (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16: 2346-2353.
- Kaiser JP, DienerL, WickP (2013) Nanoparticles in paints: A new strategy to protect façades and surfaces? Journal of Physics: Conference Series 429: 012036.
- Lai JCK, et al. (2008) Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. International Journal of Nanomedicine 3: 533-545.
- Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, et al. (2011) ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. ToxicolIn Vitro 25: 231-241.
- Chad EI, et al. (2009) Reactive Nanoparticles in Coatings, in Nanotechnology Applications in Coatings.American Chemical Society. pp. 188-209.
- Saber AT, Jensen KA, Jacobsen NR, Birkedal R, Mikkelsen L, et al. (2012) Inflammatory and genotoxic effects of nanoparticles designed for inclusion in paints and lacquers. Nanotoxicology 6: 453-471.
- Ronald LC, AndrewWM (2009) Nanoparticle Surface Modification for Advanced Corrosion Inhibiting Coatings, in Nanotechnology Applications in Coatings.American Chemical Society. pp. 64-88.
- Cao Z, et al. (2009) Synthesis and UV shielding properties of zinc oxide ultrafine particles modified with silica and trimethylsiloxane. Colloids and Surfaces A: Physicochemical and Engineering Aspects 340: 161-167.
- Dhoke S, MangalSinhaTJ, KhannaAS (2009) Effect of nano-Al2O3 particles on the corrosion behavior of alkyd based waterborne coatings. Journal of Coatings Technology and Research 6: 353-368.
- Dhoke SK,KhannaAS (2009)Electrochemical behavior of nano-iron oxide modified alkyd based waterborne coatings. Materials Chemistry and Physics 117: 550-556.
- Nakajima A, et al. (2000) Preparation of hard super-hydrophobic films with visible light transmission. Thin Solid Films 376: 140-143.
- Dyett BP, WuAH, LambRN (2014) Toward Superhydrophobic and Durable Coatings: Effect of Needle vs Crater Surface Architecture. ACS Applied Materials & Interfaces 6: 9503-9507.
- Wankhede RG, et al.(2013) Development of Hydrophobic Non-Fluorine Sol-Gel Coatings on Aluminium Using Long Chain Alkyl Silane Precursor.
- Wankhede RG, et al. (2013) Development of water-repellent organic–inorganic hybrid sol–gel coatings on aluminum using short chain perfluoro polymer emulsion. Applied Surface Science 283: 1051-1059.
- Wankhede RG (2014) Development of hydrophobic inorganic-organic hybrid sol-gel coatings on aluminium using nano-particles, in Metallurgical and Materials Science.IITB Monash Research Academy.
- KasturiJanakiVarun ASK (2015) Development of low surface energy foul release coatings for ships, in Department of Metallurgical Engineering and Materials Science.Indian Institute of Technology: Mumbai, India.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences