Green Biological Synthesis, Properties of Magnetite Nanoparticles (Fe3O4 Nps) Using Qazwan Seeds Extract as Antimicrobial Agent
Roonak Golabiazar1*, Zagros Abdulrahman Omar1, 2 and Karwan Ismael Othman3
1Department of Chemistry, Soran University, Faculty of Science, Soran, Kurdistan Regional Government (KRG), Iraq.
2Department of Pharmacy, Soran University, Rwandz Private Technical Institute, Rawanduz, Soran, KRG, Iraq
3Department of Biology, Soran University, Faculty of Science, Soran, KRG, Iraq
- *Corresponding Author:
- Roonak Golabiazar
Department of Chemistry, Soran University, Faculty of Science, Soran,
Kurdistan Regional Government (KRG),
Iraq,
Email: rgolabiazar@yahoo.com
Received date: July 05, 2022, Manuscript No. Ipnto-22-2598; Editor assigned date: July 07, 2022, PreQC No. Ipnto-22-2598 (PQ); Reviewed date: July 18, 2022, QC No. Ipnto-22-2598; Revised date: July 26, 2022, Manuscript No. Ipnto-22-2598 (R); Published date: Aug 05, 2022, DOI: 10.36648/2471-9838.8.8.92
Citation: Golabiazar R, Omar ZA, Othman KI (2022) Green Biological Synthesis, Properties of Magnetite Nanoparticles (Fe3O4 Nps) Using Qazwan Seeds Extract as Antimicrobial Agent. Nano Res Appl Vol.8 No.8: 92.
Abstract
This research reports the green and environmentally friendly preparation of the magnetite nanoparticles (Fe3O4 NPs) using aqueous extract of Qazwan (Pistacia atlantica) seeds. The green Fe3O4 NPs were characterized with different characterization techniques such as Ultraviolet-Visible (UV-vis) Spectrometer, Energy Dispersive X-Ray Diffraction (XRD), Transmission Electron Microscopy (TEM), and Field Emission Scanning Electron Microscopy (FESEM) equipped with Energy Dispersive Spectroscopy (EDS), and Fourier Transform Infrared (FTIR) spectrophotometer. The XRD, TEM, and SEM demonstrate the generation of crystalline green Fe3O4 NPs with mean diameter of 14-15 nm. The UV-Vis spectrum and FTIR were used to identify the biomolecules in the Qazwan seed extract which act as capping, reducing and efficient stabilizer agent for synthesized green Fe3O4 NPs. Additionally, the antibacterial activity and antifungal of biologically synthesized nanoparticles was proved against gram positive (Enterococcus faecalis), gram negative (E. coli, Pseudomonas aeruginosa, Acinetobacter baumanni and Klebsiella pneumonia)), and Candida albicans pathogenic fungi strains.
Keywords
Magnetite nanoparticles; Biomolecules; Plant extract; Qazwan (Pistacia atlantica) seeds; Capping agent; Green synthesis; Antimicrobial activity; Gram positive bacteria; Gram negative bacteria.
Introduction
Recently, researches on iron nanoparticles preparation have been widely developed and become a powerful investigation subject. Over the years, various physical and chemical methods have been developed to synthesize INPs which are usually expensive and potentially harmful to human health and the environment. In contrast to the conventional chemical and physical methods, green synthesis INPs has many benefits such as facile, simple manufacturing procedure, fast, economic, less waste production and easy to scale-up for industrial productions. Currently, many researchers have reported synthesis of INPs by using biological systems, including plants algae and different microorganism (yeast, fungi, diatoms, and bacteria). However, biosynthesis using plant extract is the most popular among them all. Interestingly, plant extracts are full of bioactive compounds such as ketones, aldehydes, polyphenols, caffeine, and carbohydrates for the green synthesis of nanoparticles and prevent the agglomeration of anchoring nanoparticles. The plant extract can act as a natural source for reducing, stabilizing and capping agent in the synthesizing process of nanoparticles in ambient temperature and atmosphere. While, chemical synthesis of INPs is usually done at high temperatures and under protected atmosphere. Furthermore, plant extract synthesis of INPs is one of the approaches that improve physicochemical and biological properties of nanoparticles.
Among INPs, magnetite nanoparticles (Fe3O4 NPs) exhibit unique and useful magnetic properties for a wide range of applications. The size distributions shape, biodegradable, non-toxic to human, and biocompatibility of the Fe3O4 particles are of paramount importance for medical application. These characteristics show a great potential of Fe3O4 NPs in drug delivery, magnetic targeting, hyperthermia, thermal-ablation, stem cell sorting and manipulation, gene therapy, negative MRI contrast enhancement, bioprocess intensification, antimicrobial agents, bioseparation, Li-ion batteries, ferro-fluid technology, environmental remediation, and pigments.
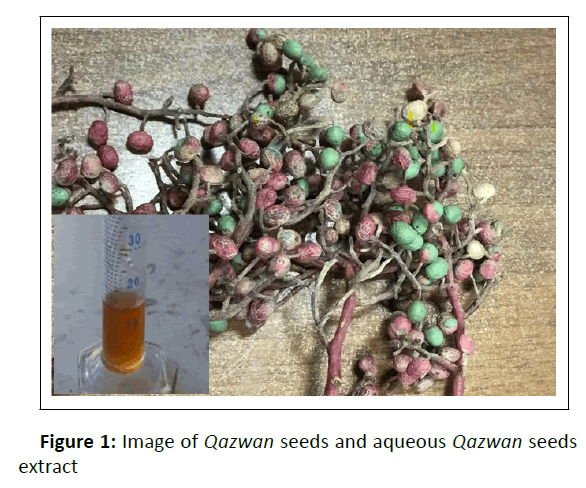
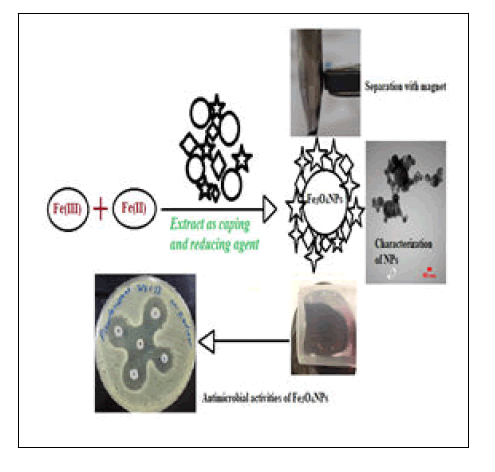
In this study, Fe3O4 NPs were successfully synthesized using Qazwan (originated from Rawanduz region of Kurdistan Regional Government, Iraq) seeds extract as a reductant and capping agent (Figure 1). Qazwan (Pistacia atlantica) is found widely in the Zagrous Mountains, and particularly in western and northern Iran, eastern and northern Iraq, southern Turkey and northern Syria in so-called Kurdistan. In previous studies, Qazwan seeds extract was reported contains alkaloids, α-Pinene, and flavonoids, which were major factors for preparation and prevent Fe3O4 NPs agglomeration here. However, the Qazwan seeds extract has been used as a reducing and stabilizing agent for the precursor iron salts through an eco-friendly, simple and cost-effective green method. Finally, its antibacterial and antifungal activity was investigated by the disk diffusion method for gram-positive (Enterococcus faecalis), gram negative (E. coli, Pseudomonas aeruginosa, Acinetobacter baumanni and Klebsiella pneumonia)), and Candida albicans pathogenic fungi strains.
Experiment
Materials
All chemical reagents used as starting materials were of analytical grade and purchased without any further purification. Ferric chloride hexahydrate (FeCl3.6H2O, 98%), ferrous (II) solfate heptahydrate (FeSO4.7H2O), Ethanol (C2H5OH) were purchased from SD fine chemicals Pvt. Ltd., India company. The Qazwan seeds used in this study originated from Rawanduz region of Kurdistan Regional Government (KRG), Iraq. Distillated deionized water was used during this research. We have taken several bacterial species, gram positive (Enterococcus faecalis (ATCC29212)), gram negative (Escherichia coli (E. coli) (ATCC25922), Pseudomonas aeruginosa (ATCC27853), Acinetobacter baumanni (ATCC19606) and Klebsiella pneumonia (ATCC13048))), and Candida albicans (ATCC10231) pathogenic fungi strains from the Department of Biology, Soran University in KRG, Iraq.
Preparation of the plant extract and green synthesis of Fe3O4 NPs
Dried and powdered seeds of the plant (about 20 g) were transferred into a 500 ml Erlenmeyer flask with 100 ml of distilled water along with boiling at 80°C for 1hr. The aqueous extract cooled down to room temperature, then was filtered and stored at 4°C as a stock for the synthesis of Fe3O4 NPs. In a typical synthesis of Fe3O4 NPs, 25 ml of extract was added dropwise to 50 ml of aqueous solution of iron salts (FeCl3 and FeSO4 with a 2:1 M ratio) under stirring for 10 min. into the continuously stirred mixture, the pH solution was adjusted about 3. Synthesized Fe3O4 NPs exhibit light red to dark brown color.
Screening of antibacterial and antifungal activities
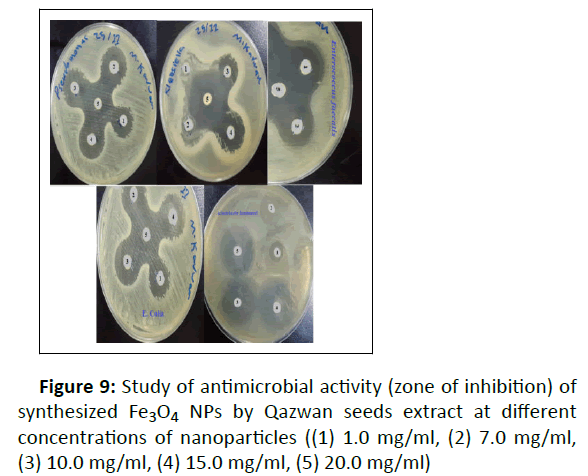
The disc diffusion technique was used. About 300 μl of microbes cultures of age 24 h were added to Petri plates and nutrient agar was poured. Once the medium was solidified, holes were made and each hole was packed with different concentrations of nanoparticles ranging from 1.0 to 20.0 mg/ml. The plates were wrapped in parafilm tape and transferred to incubator and maintained at 37°C for 24 h. The inhibition zones of were then recorded in millimeters.
Measurement techniques
The magnetite green nanoparticles (Fe3O4) were characterized FESEM, TEM, EDX, XRD, FTIR, and Uv-Vis spectrometry. The cross section morphology of the nanoparticle (Fe3O4) was studied by Field emission FESEM (Quanta 450) equipped with EDS (Quantax EDS features the XFlash® 6 detector) at accelerating voltage 20 kV. A thin layer of gold was coated on the all samples before microscopic analysis. Bright field TEM images are recorded on a (Philips EM 120) transmission electron microscope at an accelerating voltage of 80 kV. The samples for TEM analysis are cut into slices of a nominal thickness of 100 nm using an ultra-microtome with a diamond knife on a Rechert Ultracut ultra-microtome at ambient temperature. The cut samples are supported on a copper mesh for this analysis. The X-ray diffraction (XRD) patterns of the sample was recorded at room temperature on a Philips powder diffractometer type (PW1373 goniometer) using Cu Kα (λ = 1.54060 Å) radiation with scanning rate of 2° min-1 in the 2ϴ range from 0° to 75°. Scanning was made for the selected diffraction peaks which were carried out in step mode (step size 0.01°, measurement time 0.5 seconds, accelerating voltage of 45 KV an emission current of 40 mA measurement). UV–Vis spectra of samples were recorded using light source versatile lamps (Carry 100, tungsten halogen light sources). The presented results were obtained at room temperature. The binding properties of nanoparticles synthesized by co-precipitation method were investigated by FTIR spectroscopy (IRAffinity-1 Shimadzu Corp. A213750). Dried and powdered nanoparticles were pelleted with potassium bromide (KBr). The spectra were recorded in the wavenumber range of 400–4000 cm−1 and analyzed by subtracting the spectrum of pure KBr.
Results and Discussion
Characteristic of the magnetite nanoparticle
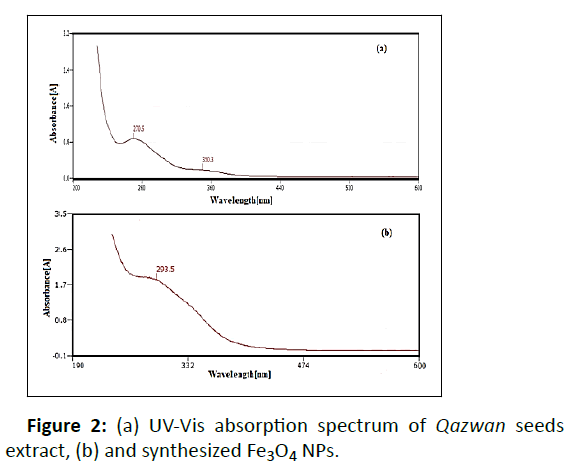
In this study, we describe the using of biosynthesis method for the synthesis of Fe3O4 NPs using Qazwan seeds extract as a reducing and stabilizing agent. As can be seen in Figure 2a, the UV-Vis spectrum of Qazwan seeds extract shows absorption double bonds at λmax = 350 (bond I) and 270 nm (bond II). They are related to the transition within C-C system of aromatic and C=O bonds. The effect of surface plasmon resonance on the color change for green synthesized Fe3O4 NPs shows its absorbance maxima (λmax around 293 nm) which demonostrating the formation of Fe3O4 NPs (Figure 2b).
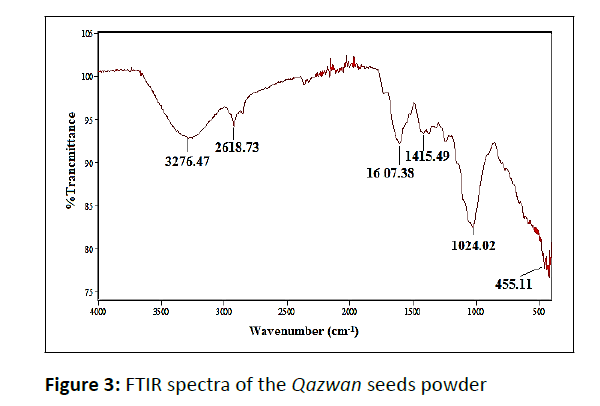
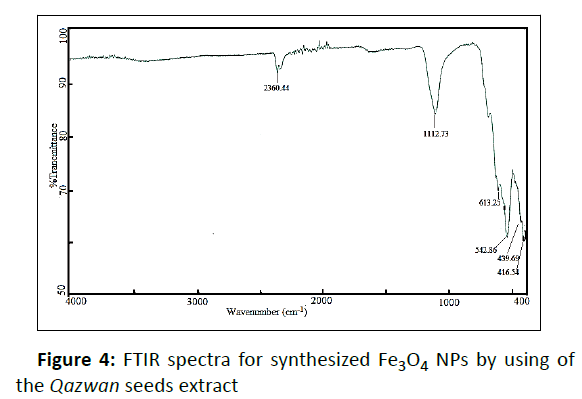
As can be seen in the FTIR spectra of Qazwan seeds (Figure 3), the major peaks around 1024 cm−1 (C−O stretch), 1415 cm−1 (stretching C=C aromatic ring), 1607 cm−1 (C=O stretch and N−H bending), 2918 cm−1 (C−H stretch), and 3000–3600 cm−1 (O−H stretch) were observed. Therefore, the obtained results by FTIR and UV-Vis (Figure 2a) analysis support the reported results in previous literature, indicating the presence of flavonoid and phenolic antioxidants inside the plant extract. In fact, the antioxidant activity plants are attributed to the redox potential of phytochemicals to quench singlet and triplet oxygen, decompose peroxides or neutralize free radicals. Furthermore, the FTIR of Fe3O4 NPs synthesized by the plant extracts (Figure 4) shows demonstrative differences in the shape and location of signals due to the interaction between Fe ions and involved sites of biomolecules for the capping and efficient stabilization of nanoparticles. These all confirm that extract is absorbed in the surface of Fe3O4 NPs. In addition, the strong broad peak at 542 cm-1 is due to the stretching vibrations of Fe-O bond.
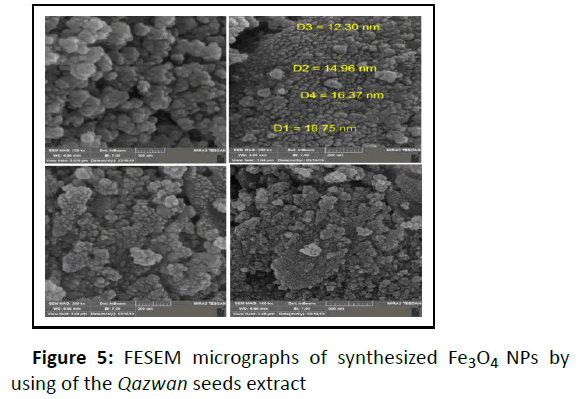
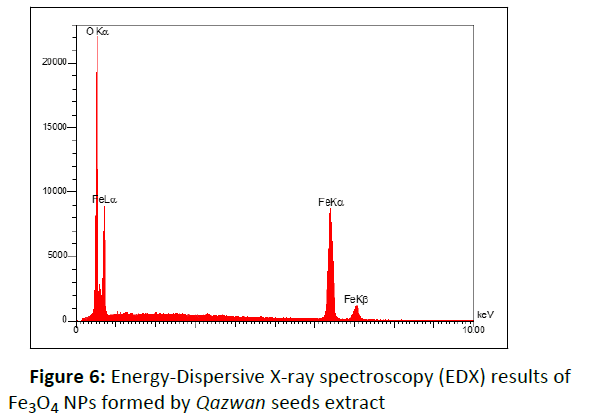
Size, shape and surface morphology of the nanoparticles were determined by Scanning Electron Microscopy (SEM). The FESEM images of Fe3O4 NPs in (Figure 5) show the spherical particles with an average diameter of 12 to 18 nm. The elemental composition of the Fe3O4 NPs was also analyzed by the Energy Dispersive X-ray Spectroscopy (EDX). The existence of the strong signals with the highest percentage of Fe (52.06%) and O (47.94%) in EDX spectrum (Figure 6) confirmed the formation of Fe3O4 NPs.
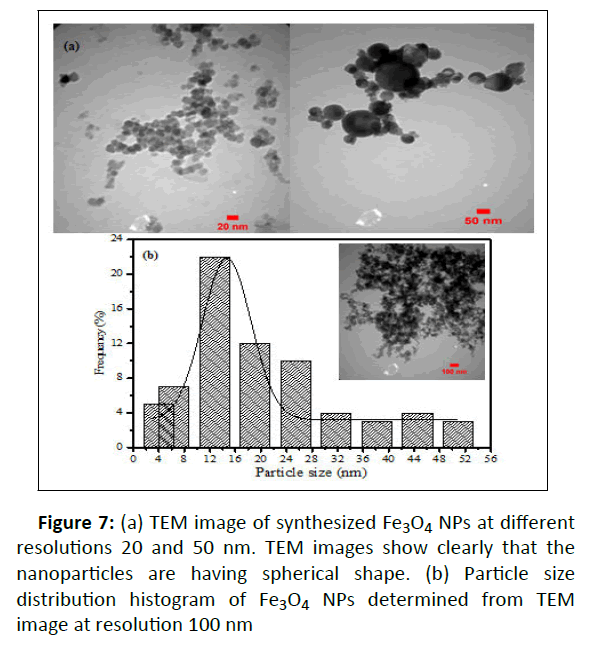
As can be seen in Figure 7a, the TEM micrographs show spherical shape and polydispersed of green synthesized Fe3O4 NPs. A particle size distribution histogram was measured from the TEM image at resolution 100 nm as shown in Figure7b. The histogram clearly defined the average hydrodynamic size of the Fe3O4 NPs to be in the range of 2 to 54 nm with 14.65 nm mean diameter.
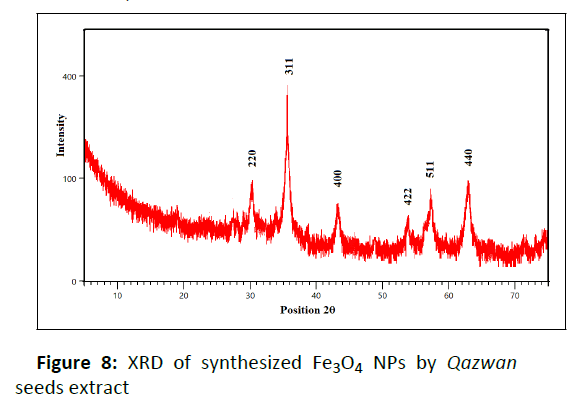
Phase purity and crystallinity of Fe3O4 NPs synthesized by the Qazwan seeds extract is shown in Figure 8. The XRD signals of Fe3O4 NPs at 30.26˚, 35.60˚, 43.26˚, 53.95˚, 57.32˚and 62.94˚ represent the crystalline planes (220), (311), (400), (422), (511) and (440), respectively, which obviously show its crystalline cubic structure of magnetite phase (Fe3O4) (JCPDS file no: 00-003-0863).
The crystallite size of the nanocrystalline samples was measured from the X-ray line broadening analyses using Debye- Scherrer formula a ter accounting for instrumental broadening (Eq.1).
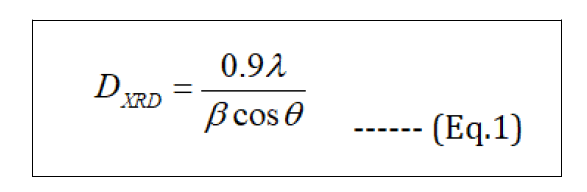
Where DXRD - crystallite size in nm, λ- wavelength of X-ray radiation for Cu Kα (0.15406 nm), β – is the line broadening at half the maximum intensity (FWHM in radians in the 2θ scale), θ - the Bragg angle. Using the equation, the average of the particle size of synthesized Fe3O4 NPs was found to be 14.22 nm, which was calculated from the full-width at half maximum of (311) plane at 2θ = 35.60°. Dislocation density (∂) is calculated with the DXRD - crystalline size (Eq.2). The values obtained are shown in Table 2.
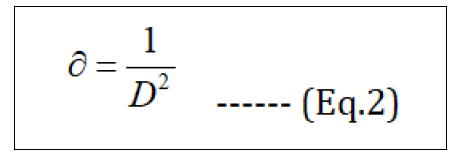
Micro strain is calculated by the formula;
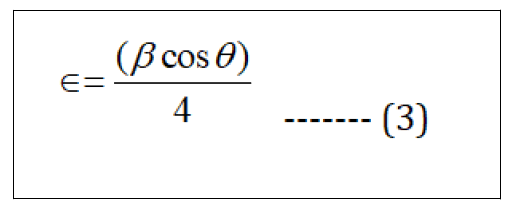
The lattice parameter "a" and interplanar spacing dhkl are determined by Bragg's law (Eq.4) and (Eq.5).
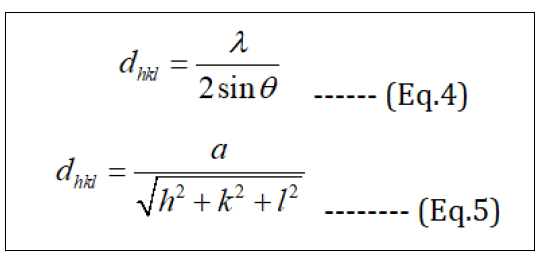
These results in Table 1 show that dislocation density and micro strain have similar behaviour as decreased with the increase in the crystallite size. Also, the lattice parameter and interplanar spacing for the synthesized Fe3O4 NPs is (a = 8.3519 A˚ and d311 = 2.5218), respectively. The estimated values for (a and d311) lower than the values reported for bulk magnetite JCPDS Card No. (79-0417) (a = 8.394 and d311= 2.531), but these values are closed to the values in some references.
| Observed | Observed d(A°) | Crystalline Size(nm) | Dislocation density (∂) | Strain (ε) | h | k | l |
|---|---|---|---|---|---|---|---|
| 2q (°) | a = 8.3519 A° | (×10-3) lines/m4 | |||||
| (×1015) lines/m2 | |||||||
| 30.2636 | 2.95333 | 19.8208 | 2.54 | 0.745 | 2 | 2 | 0 |
| 35.6016 | 2.5218 | 50.4908 | 3.92 | 0.24973 | 3 | 1 | 1 |
| 43.2651 | 2.09123 | 31.2364 | 1.02 | 0.33474 | 4 | 0 | 0 |
| 53.951 | 1.69956 | 28.846 | 1.2 | 0.29459 | 4 | 2 | 2 |
| 57.3259 | 1.60727 | 26.2883 | 1.44 | 0.3057 | 5 | 1 | 1 |
| 62.9428 | 1.47669 | 16.7639 | 3.55 | 0.44044 | 4 | 4 | 0 |
Table 1: The values of observed‘d’, crystalline size, dislocation density, strain and h, k, l of Fe3O4 NPs
Antimicrobial activities of magnetite nanoparticle
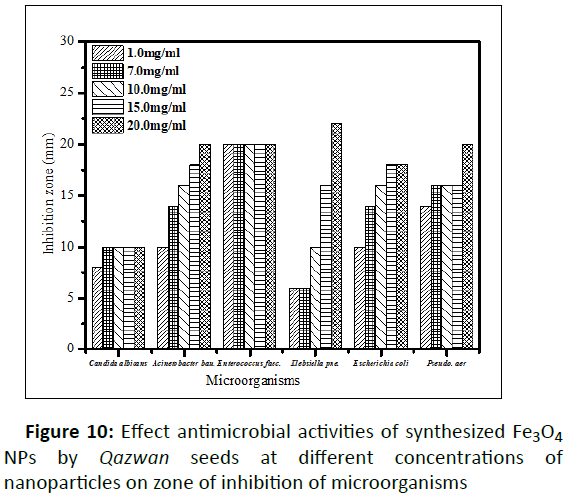
In this study, the antimicrobial activities of the green Fe3O4 NPs evaluated against several pathogenic bacteria and fungi in different concentration of nanoparticles. For this purpose, antibacterial and antifungal effect of the Fe3O4 NPs are presented on gram positive (Enterococcus faecalis), gram negative (E. coli, Pseudomonas aeruginosa, Acinetobacter baumanni and Klebsiella pneumonia)), and Candida albicans pathogenic fungi strains in the attendance of different Fe3O4 NPs concentrations (1.0, 7.0, 10.0, 15.0, and 20.0 mg/ml in H2O) in (Table 2 and Figure 9 to 10). The zone of the clearance around each well after the incubation period, confirms the antimicrobial and antifungal activity of the green Fe3O4 NPs (Figure 9). An increase in the size of inhibition zone was noticed for all the bacteria tested with increasing concentration of Fe3O4 NPs. As can be seen of Figure 10 and Table 2, the higher concentration of Fe3O4 NPs demonstrated a better antibacterial potential for gram negative against the gram positive bacteria. The higher bioactivity of the green Fe3O4 NPs could also be attributed to the large surfaces of nanoparticles and preferential adsorption of the bioactive constituents from Qazwan seeds extract onto the surface of the Fe3O4 NPs. According to literature, when these nanoparticles with higher surface area and smaller size than the bacteria membrane pores enter the bacterial cell and interact with sulphur containing proteins and phosphorus containing DNA, they disrupt the respiratory chain and cell division.
| Bacterial strains | Concentration of Fe3O4 NPs (mg/ml) | ||||
|---|---|---|---|---|---|
| 1.0 mg/ml | 7.0 mg/ml | 10.0 mg/ml | 15.0 mg/ml | 20.0 mg/ml | |
| Zone of inhibition (mm) | |||||
| Escherichia coli | 10 | 14 | 16 | 18 | 18 |
| Acinetobacter baumannii | 10 | 14 | 16 | 18 | 20 |
| Pseudomonas aeruginosa | 14 | 16 | 16 | 16 | 20 |
| Klebsiella pneumonia | 6 | 6 | 10 | 16 | 22 |
| Enterococcus faecalis | 20 | 20 | 20 | 20 | 20 |
| Candida albicans (fungi) | 8 | 10 | 10 | 10 | 10 |
Table 2: Zone of inhibition for microorganisms in the presence of different Fe3O4 NPs concentrations at room temperature
Graphical Abstract
Conclusion
Magnetite nanoparticles (Fe3O4 NPs) were synthesized using a green biosynthetic method by the biological materials found in the Qazwan seeds extract. Biomaterials in the Qazwan seeds extract which act as capping; reducing and efficient stabilizer agent synthesized the Fe3O4 NPs by reducing the precursor iron salts. The existence of the strong signals with the highest percentage of Fe and O in EDX spectrum together, XRD, TEM, and FESEM images confirmed the formation of crystalline green Fe3O4 NPs with mean diameter of 14-15 nm. The biosynthesized Fe3O4 NPs by Qazwan seeds extract is an eco-friendly, simple and cost-effective green method against chemical methods. This advantageous can be a promising development because of the lack of toxic chemicals usage and required only non-hazardous reactants like plant extract. Additionally, green Fe3O4 NPs with higher surface area and smaller size than the bacteria membrane pores cross them and show a significant effect on gram positive, gram negative bacteria and fungi strains.
Acknowledgments
The authors would like to thank research center at Soran University in Iraq for taken the XRD, research center at Arya Electron Optic, and Tehran University in Iran for taken (FESEM, EDX, and TEM) respectively. Also Faculty of Biology at Soran University for assistance with antimicrobial tests and for the constructive discussions.
Conflict of Interest
The authors report no conflict of interest in this work.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences