Preparation and Characterization of Magnetic Glass Ceramics Derived from Iron Oxides Bearing Rolling Mill Scales Wastes
Salwa AM Abdel-Hameed, Ibrahim Hamed M, Barbara J Muller-Borer, Nehal A Erfan
Salwa AM Abdel-Hameed1, Ibrahim Hamed M2, Barbara J Muller-Borer3, Nehal A Erfan2*
Glass Research Department, National Research Centre,33El Bohouthst.(former El Tahrirst.)-Dokki-Giza- P.O.12622 , Egypt
Chemical engineering department, faculty of engineering, Minia university, Minia, Egypt
Department of Engineering, East Carolina University, Greenville, NC, USA
- *Corresponding Author:
- Nehal A Erfan
Chemical engineering department, faculty of engineering, Minia university, Minia, Egypt.
Tel: +1-252-412-1154
E-mail: erfann14@ecu.edu
Received date:September 02, 2015, Accepted date:October 27, 2015, Published date: November 05, 2015
Citation: Abdel-Hameed SAM, Hamed IM, Muller-BorerBJ, et al. Preparation and Characterization of Magnetic Glass Ceramics Derived from Iron Oxides Bearing Rolling Mill Scales Wastes. Nano Res Appl. 2015, 1:1.
Abstract
Rolling Mill Scales (RSW) were used as raw materials for preparation of hard and soft magnetic glass ceramics which have a wide range of applications. In steel processing up to 5% of steel is lost with scales during the hot rolling operation where Iron oxides form~ 69-72% of the milling scales weight. In addition to the iron content, mill scales are contaminated with remains of lubricants, oils and grease from the equipment associated with rolling operations. The oil content typically ranges from 0.1 and 2% -but can reach up to 20%-beside ~10% water. The goal of this project was to evaluate magnetic properties of soft and hard magnetic glass ceramics synthesized from RSW. To synthesize soft magnetic glass ceramics (SMGC) and hard magnetic glass ceramics (HMGC), 65 wt% and 37 wt% respectively of RSW were used. Quenching-melting technique was utilized. Differential thermal analysis (DTA) showed one broad exothermic peak at 906ºC and at 731ºC for SMGC and HMGC respectively. For SMGC, the major detectable peaks belong to Znferrite( ZnFe2O4) and Hematite(Fe2O3) as shown by X-ray diffraction (XRD) analysis. Alternatively, Ba-hexaferrite (BaFe12O19) and Hematite are the detected phases in HMGC samples. After heat treatment, crystallization of ~131-166 and 24-34 nm magnetic particles were detected for SMGC and HMGC via transmission electron microscopy (TEM). An increase in saturation magnetization from 18 emu/g for RSW to 66.5 emu/g for heat treated SMGC and 19 emu/g for HMGC was measured with a Vibrating Scanning Magnetometer (VSM).The results confirmed that, soft and hard magnetic glass ceramic nanoparicles based on RSW with magnetic phase content reaches 92.4 wt% was obtained.
Keywords
Rolling mill scales; Glass-ceramics; Nanoparticles; Ferrimagnetism; Znferrite and Ba-hexaferrite
Introduction
The properties of nanomaterials were not well understood although they have been in use for centuries. Early Chinese dynasty porcelain glazes were the earliest application [1]. During the Renaissance period, artists in Umbria, Italy utilized nanomaterials in art fabrication [2]. Several decades ago, serious research on nanotechnology began [1]. Since then, significant improvements have been made on process development of nanomaterials with controlled structure and properties [1]. Recently, one-dimensional (1D) and quasi-one-dimensional (Q 1D) materials with magnetic phases have attracted great attention due to their potentials as building blocks for future electromagnetic devices [3,4].
The need for wastes recycling is important as scientists continue to seek ways to reduce the environmental impact of industrial wastes. Industrial waste has been a major source of air, water and industrial pollution resulting from solid waste disposal. This consequently has a negative impact on the environment with increased associated costs. Owing to the growing amount of solid wastes produced by industrial firms, increased environmental regulations and the need for pollution abatement, there is increased interest in using recycling as a means of diverting solid wastes to useful products as glass-ceramics.
The following are examples for producing glass ceramics from solid wastes. Blast-furnace slag was the first silicate waste to be investigated as a source material for glass ceramics. These slags consist of CaO, SiO2 and MgO as the main constituents, with minor constituents such as MnO, Fe2O3 and S. The first attempt to commercialize a glass ceramic from slag was by the British Iron and Steel Research Association in the late 1960’s [5]. Fabrication of glass-ceramics using slag-type wastes from non-ferrous metal production has been reported, these include copper slag [6,7] and phosphorus slag [8]. Up to 40 wt% of copper slag was incorporated into a base glass composition to produce tiles via the powder sintering method [6]. Mixtures of coal ash and waste glass have been used in early technological approaches to the fabricate of glass ceramics and glass matrix composites using powder technology and sintering [9-11]. Cheng et al. conducted single-step heat treatment of glasses obtained from vitrified incinerator fly ash [12] and of a mixture of electric arc furnace (EAF) dust and fly ash [13].
M Pelino et al. [14] and Morsi et al. [15] prepared glass specimens by melting mixtures of magnesite, feldspar, quartz sand, kaolin and cement dust with dust content in the range 25–37 wt%. Gorokhovsky et al. [16] produced diopside-based glass ceramics based on a combination of a wide range of wastes (quartz-feldspar waste, limestone dust, phosphorus slurry, metallurgical slag) and selected commercially available chemicals, such as Cr2O3, as a nucleating agent. Yun et al. [17] prepared glass-ceramics from a mixture of fluorescent glass and waste shell in a weight ratio of 4:1. Diaz et al. [18] recently reported that it is possible to produce cordierite glass-ceramics from inorganic wastes of anodizing plants .Reusing jarosite and goethite from hydrometallurgical processes to obtain glass and glass-ceramic materials has been reported in several papers [19-25].
To our knowledge, production of magnetic glass ceramics from rolling mill scales has not been previously reported.
Rolling mill scales are solid by-products from the steel making industry that contain metallic iron and three types of iron oxides: Wustite, Hematite and magnetite. Rolling mill scales also contain traces of non-ferrous metals, alkaline compounds and oils from the rolling process [26]. Mill scales are formed on the outer surfaces of plates, sheets or other configurations produced by rolling red-hot iron or steel billets in rolling mills. Mill scales with bluish black color are composed of iron oxides mostly ferric (Figure 1). In the whole world, 13.5 million tons of mill scales are generated annually [27]. Depending on the process and the nature of the product, the weight of mill scale can vary between 20 and 50 kg/t of the hot rolled product. The average specific production of this by-product is typically 35-40 kg/t [28]. Approximately 90% of mill scales is directly recycled within the steel making industry and small amounts are used for ferroalloys, in cement plants and in the petrochemicals industry [29-32]. Volodymer et. al. [26] utilized the rolling mill scales in the iron ore sintering process as they were able to improve the combustion of the scale’s oil at the sintering process by preparation of a mixture with peat. By their method the consumption of the oiled scale was increased from zero to 12.8 kg. A study was made by MI Martin et. al. [28] of the reduction of mill scale to sponge iron using coke at different temperatures. N.M. Gaballah et. al., [33] prepared two models for the production of iron from rolling mill scales wastes via hydrogen reduction at varying temperatures. Reintroducing the rolling mill scales into an industrial process through well planned programs will reduce their environmental impact and will improve cost savings.
Glass-ceramics are defined as composite materials that contain at least one crystalline phase dispersed in an amorphous glassy matrix. The chemical composition of the glass precursor influences the physical and chemical properties of the composite. Therefore, the investigation of new composite glass materials with controlled particles size distribution and aspect ratio has an impact on industry. Preparation of magnetite-containing glass ceramics has been reported by several groups [34-36] where ferromagnetic bio glass-ceramics containing 45 wt% of magnetite were prepared [37] and about 60 wt% were reported by others [38,39]. Traditional compact magnetic materials are commonly prepared by sintering of ferrite powders [40,41] with a mean particle size of about 1 μm at 1200-1250°C. During sintering the magnetic crystals grow so that multi-domain behavior occurs which lowers the coercivity of the material [42].
Spinel and hexa-ferrites are a large important class of materials with the general formula M-Fe2O4 for spinel ferrites and M-Fe12O9 for hexa-ferrites, where M is a divalent cation.Hexa-ferrites have a hexagonal crystal structure where the magnetic properties of the spinel ferrites originate mainly from the magnetic interactions between cations with magnetic moments, which are situated in tetrahedral and octahedral sites [43-45]. The prepared materials have wide spread applications in material science [40,46-51] and biotechnology [38,39,52].
In this study, glass techniques were used to prepare a compact body in which magnetic particles were well dispersed and kept in single domain behavior. We prepared compact glass ceramics that contain single domain particles (<100 nm) separated by a nonmagnetic matrix which lowers the sinter temperature, prevents the particles from growing to be in multi-domain and resulted in low magnetic interaction. The main objective of this research was to evaluate RSW from various metallurgical industries and to prepare soft and hard nanomagnetic glassceramics.
These nanomagnetic glass-ceramics have potential applications in health care such as cell separation, magnetic resonance imaging contrast agents [53], drug delivery, tissue engineering and hyperthermia treatment of cancer.In addition, they have a wide range of applications in information storage systems [54], ferrofluid technology [55,56], magnetocaloric refrigeration, gassensors and catalysis [57].
Experimental Methods
Preparation of the glass
X-Ray Fluorescence (XRF) analysis was used to determine the composition of the RSW samples as illustrated in Table 1. The samples were prepared with two compositions: one based on crystallization of ZnFe2O4 as a soft magnet and the other based on BaFe12O19 as a hard magnet. The compositions of the prepared samples are reported in Table 2. RS and RH are soft and hard magnetic reference samples prepared from pure chemicals such as ZnCO3, Na2CO3, NH4H2PO4, TiO2, BaCO3, H3BO3 and SiO2. SMGC refer to soft magnetic glass ceramics that contain about 65% of RSW while HMGC to hard magnetic glass ceramics that contain~37% of RSW. Small amounts of ZnO as ZnCO3, Na2O as Na2CO3, P2O5 as NH4H2PO4, TiO2, BaO as BaCO3 and B2O3 as H3BO3 were added to complete the design of the desired phases.As previously described (Salwa A. M. Abdel-Hameed et al. 2014) the samples were prepared by melting the required amounts of reagent grade chemicals with composition shown in Table 2. Samples were prepared in platinum crucibles at 1500ºC for soft ferrite samples and 1200ºC for hard ferrites, and heated for 2 h in electrically heated furnace with occasional swirling every 30 min to ensure homogenization. The melts were poured onto a stainless steel plate in 1-2 mm thick strips by applying another cold steel roller.
| Main Constituents | (wt,%) |
|---|---|
| SiO2 | 1.33 |
| TiO2 | 0.03 |
| Al2O3 | 0.45 |
| Fe2O3tot. | 95.56 |
| MgO | 0.15 |
| MnO | 0.82 |
| CaO | 1.22 |
| Na2O | 0.11 |
| K2O | 0.05 |
| P2O5 | 0.06 |
| Cl | 0.048 |
| CuO | 0.034 |
| NiO | 0.012 |
| Cr2O3 | 0.049 |
| V2O5 | 0.038 |
| ZnO | 0.049 |
Table 1 Quantitative analysis(XRF) of rolling mill scales waste raw material(RSW).
Characterization
To determine the temperatures of the glass transition (Tg) and crystallization (Tc) of the glass samples differential thermal analysis (DTA; SDTQ600) under inert gas with a heating rate of 10oC/min was used. Alumina was used as the inert reference material. The results obtained served as a guide for determining the required heat-treatment temperatures needed to induce crystallization in the samples. To investigate the effect of heat treatment on the phase transformation and sample properties, samples were heat treated at 900oC for 2 h.Samples before and after heat treatment were evaluated using powder X-ray diffraction using a Ni-filled Cu- Kαtarget to identify their content and the precipitated crystalline phases. X-ray diffraction (XRD) was performed using Bruker D8 Advanced instrument (Germany D8 ADVANCE Cu target 1.54 A, 40 KV, 40 mA). The reference data for the interpretation of the XRD patterns was obtained from ASTM X-ray diffraction card files. Samples were crushed and sonically suspended in ethanol. A few drops of the suspended solution was placed on an amorphous carbon film held by a copper micro grid mesh. The microstructure and crystallite size was characterized using a TEM 2010 transmission electron microscope. Chemical composition of the crystals in the glass ceramics were analyzed by EDX using Oxford instrument INCAX-sight on a Joel TXA-840A electron probe micro analyzer. The magnetic properties were evaluated with a vibrating sample magnetometer (VSM, 9600-1 LDJ, USA) in a maximum applied field of 20 KOe. Saturation magnetization (Ms), remanance magnetization (Mr) and coercivity (Hc) were determined from the hysteresis loops.
Results and Discussions
Differential thermal analysis
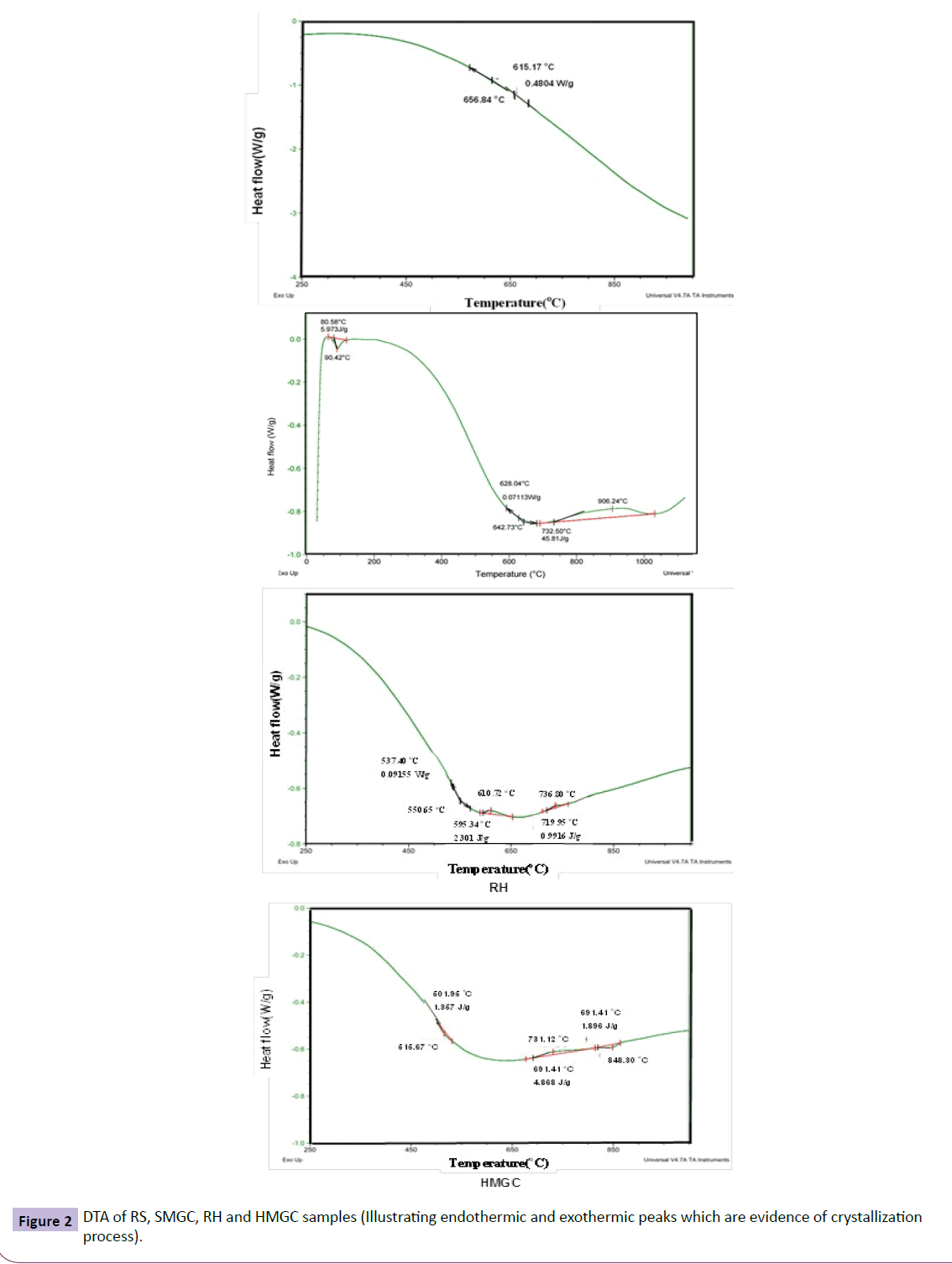
The DTA scan provides a rapid method to investigate the crystallization nature, the effect of heat and the temperature range of crystallization and the proper heat-treatment schedule. The limits of crystallization however,are about ± 30°C of the DTA crystallization peaks; as with powdered glass samples, surface nucleation and devitrification tend to obscure the internal structural changes of the glass [58].
DTA of RS, SMGC, RH and HMGC samples before heat treatment are shown in Figure 2. The materials resemble a crystalline state. When the amorphous state decreases, the endothermic and exothermic intensities decrease. In general, DTA results show lower intensity thermal effects due to the high degree of crystallization during cooling from melting temperature to room temperature. DTA results show an endothermic effect resembling an amorphous phase at 657ºC for RS, 642ºC for SMGC sample, 551ºC for RH and about 516ºC for HMGC sample. This endothermic reaction is believed to be caused by an increase in heat capacity due to transformation of glass from a rigid to plastic structure and the accompanied rearranging of different atoms as a precrystallization step [59]. The endothermic temperatures are lower in the batch compositions containing RSW than those of pure chemicals due to the reflux effect of different oxides present in RSW which lower the samples viscosity and Tg as shown in Table 2. The sharp endothermic effect at 90oC in SMGC was attributed to water evaporation while the broad exothermic peak at 906ºC is an indication for crystallization of both hematite and Zn-ferrite as confirmed by X- ray later. The absence of exothermic effect in RS sample indicates a high degree of crystallization during cooling from melting temperature to room temperature.
| Chemical Composition wt% | Sample ID | |||
|---|---|---|---|---|
| RS | SMGC | RH | HMGC | |
| Fe2O3 | 70.5 | 68.38 | 43.17 | 40.21 |
| SiO2 | - | 0.952 | 4.64 | 4.713 |
| TiO2 | 2.21 | 2.168 | - | 0.0126 |
| ZnO | 23.96 | 23.14 | - | |
| Al2O3 | - | 0.322 | - | 0.1863 |
| MgO | - | 0.107 | - | 0.0631 |
| CaO | - | 0.873 | - | 0.51 |
| Na2O | 2.21 | 2.23 | - | 0.463 |
| K2O | - | 0.036 | - | 0.21 |
| P2O5 | 1.1 | 1.12 | - | 0.025 |
| CuO | - | 0.024 | - | 0.0143 |
| Cr2O3 | - | 0.035 | - | 0.02 |
| MnO | - | 0.587 | - | 0.345 |
| B2O5 | - | - | 10.76 | 14.632 |
| BaO | - | - | 41.43 | 38.59 |
| Ni2O | - | 0.00859 | - | 0.00505 |
| V2O5 | - | 0.027 | - | - |
Table 2 Chemical composition in wt% of prepared magnetic glass ceramics.
Alternatively, a single exotherm appeared at 731oC for the HMGC sample and two exothermic peaks at 611oC and 737oC in RH sample corresponding to Ba-hexaferrite crystallization. The shape and intensity of the exothermic peak are a good indicator for the crystallization process; the sharper and stronger exothermic peak reflects larger and quicker crystallization, conversely, if the exothermic peak is broad, the crystallization is slow and small [60].The DTA results were used to determine heat treatment schedule which was applied to samples at 900oC for 2 h to study its effect on the crystallization process.
X-ray diffraction analysis
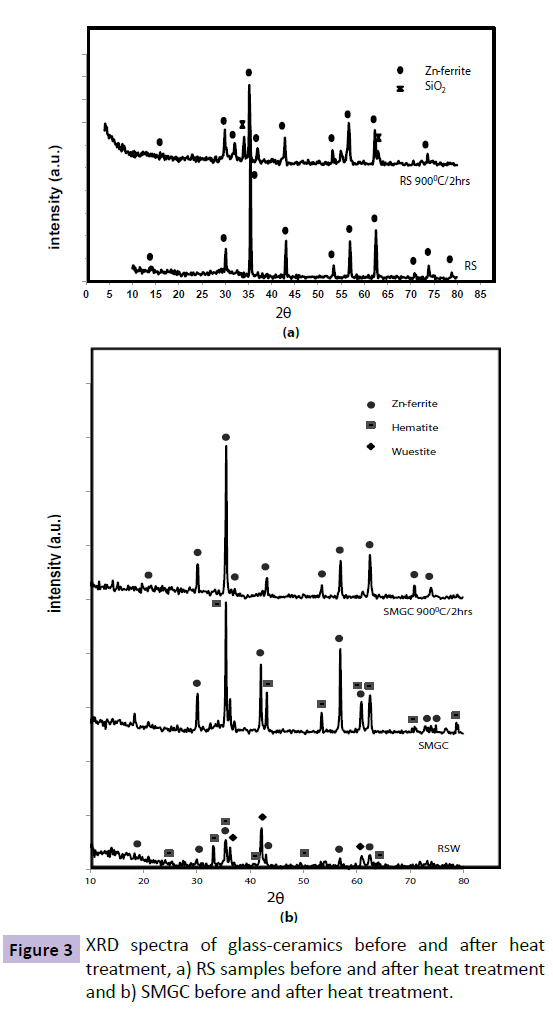
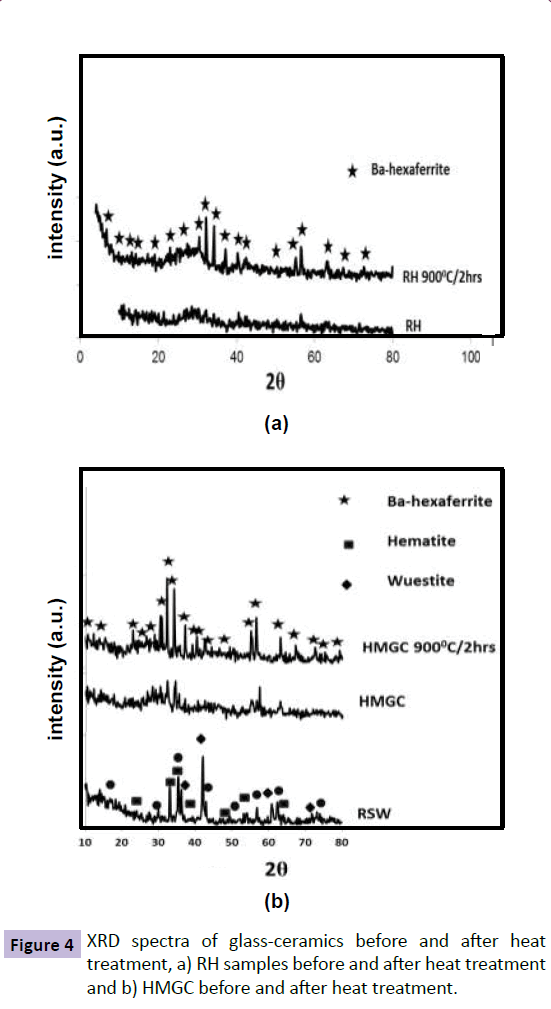
Figure 3 depict correlation between the XRD results of RSW , Rs and SMGC before and after heat treatment , While Figure 4 depict correlation between RSW,RH and HMGC before and after heat treatment.
The major detectable peaks can be indexed to Zn-ferrite (ZnFe2O4) and Ba-hexaferrite (BaFe12O19) for SMGC and HMGC samples respectively. These designed composition succeeded in reaching the designed phases. Several factors contribute to the broadening of peaks in X-ray diffraction [60,61]. For example,-factors related to the resolution and the incident X-ray wavelength of the XRD, as well as the sample crystallite size and non-uniform microstrain, can cause a line broadening. In the case of an instrumental broadening, the line width will vary smoothly with 2θ or d spacing. On the other hand, the line broadening originating from the sample characteristics will have a different relationship. Using scherrer's equation [62,63] for crystallite size and the Bragg's law for diffraction [64], crystallite size and microstrain components are estimated :
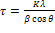
where:
•  is the mean size of the ordered (crystalline) domains, which may be smaller or equal to the grain size;
is the mean size of the ordered (crystalline) domains, which may be smaller or equal to the grain size;
• K is a dimensionless shape factor, with a value close to unity. The shape factor has a typical value of about 0.9, but varies with the actual shape of the crystallite;
λ is the X-ray wavelength;
β is the line broadening at half the maximum intensity (FWHM), after subtracting the instrumental line broadening, in radians. This quantity is also sometimes denoted as Δ(2θ);
θ is the Bragg angle.
Bragg's law is: 
where
• n is a positive integer
λ is the wavelength of incident wave
d is the spacing
Figure 3a illustrates the difference between RSW and SMGC before and after heat treatment. The RSW samples include Hematite and Magnetite phases with trace Wuestite phase which disappeared completely in SMGC. Furthermore, applying heat treatment at 900°C for 2 h affected the crystallization of Zn-ferrite as a unique phase. Comparing the main peaks in the three patterns we notice that heat treatment has a greater effect on crystallization and amount of Zn-ferrite crystallized. The XRD spectra represent the crystallization of Zn-ferrite as a single phase in RS sample (Figure 3b ), with less crystallization relative to SMGC sample. The amount of Zn-ferrite decreased in the RS sample after heat treatment at 900oC for 2 h due to the separation of SiO2 phase. Comparing the compositions of RS and SMGC, it’s evident that there are extra oxides present in SMGC sample due to RSW composition as listed in Table 2. These oxides facilitate the crystallization of Zn-ferrite by decreasing the viscosity of the sample and increasing mobility of ions to crystallize in the quenched sample.
Alternatively, Figure 4b shows the XRD spectra of HMGC before and after heat treatment. Hematite, Wuestite and Zn-ferrite phases present in the RSW sample disappear and a broad hump characterizing the amorphous glassy state appears in HMGC sample before heat treatment. Ba-hexaferrite crystallizes as the sole formed magnetic phase in a nonmagnetic matrix when the sample is exposed to heat treatment at 900°C for 2 h. A clear effect of the heat treatment on the RH sample is shown in Figure 4a, with the transformation from an amorphous to crystalline state as represented by crystallization of pure Ba-hexaferrite. More Ba-hexaferrite crystallized in HMGC than in the RH which is reflected by higher XRD peak intensities.
Transmission electron microscope
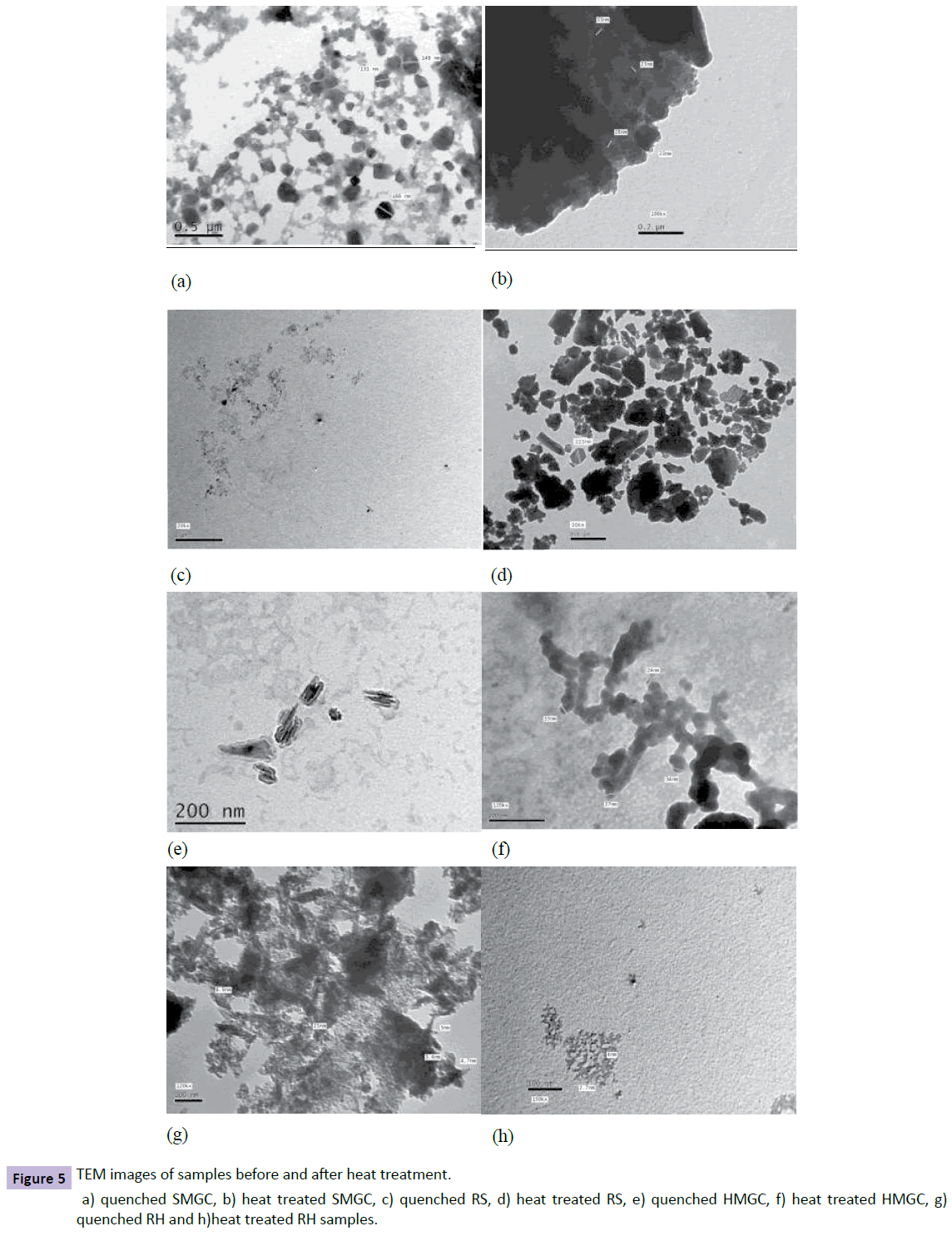
To investigate the possibility of micro twinning and the homogeneity of the materials TEM was performed. Particle size was measured from TEM images. Figure 5 shows the TEM microstructures in high magnification for SMGC, RS, HMGC and RH samples before and after heat treatment. Figure 5a,5b show the precipitation of nanosized cubic crystals of Zn-ferrite and Hematite phases in the SMGC sample before heat treatment with crystalline size range from 131-166 nm. All Hematite crystals transformed to Zn-ferrite after heat treatment with crystalline size range from 23-33 nm, this confirmed the results obtained from XRD calculations shown in Figure 3. TEM for RS samples are shown in Figure 5c, 5d which illustrates the transformation of Zn-ferrites to SiO2 phase with crystalline size of about 223 nm after heat treatment. In contrast, Ba-hexaferrite with hexagonal crystals is formed as a unique phase with crystalline size ranging from 24-34 nm in HMGC samples after heat treatment as shown in Figure 4. In addition, TEM for RH represented in Figure 5g, 5h show the crystallization of Ba-hexaferrite as a single phase with crystalline size ranging from 2-12 nm after heat treatment.
Magnetic properties
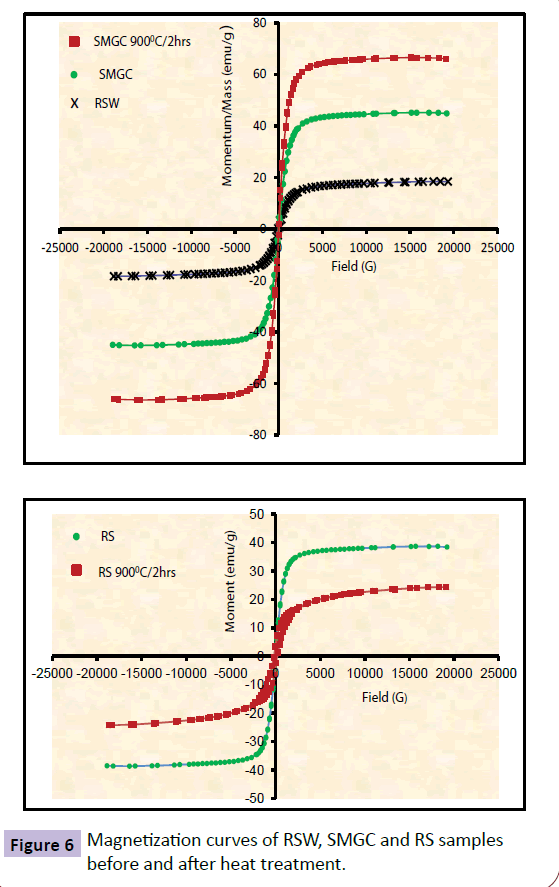
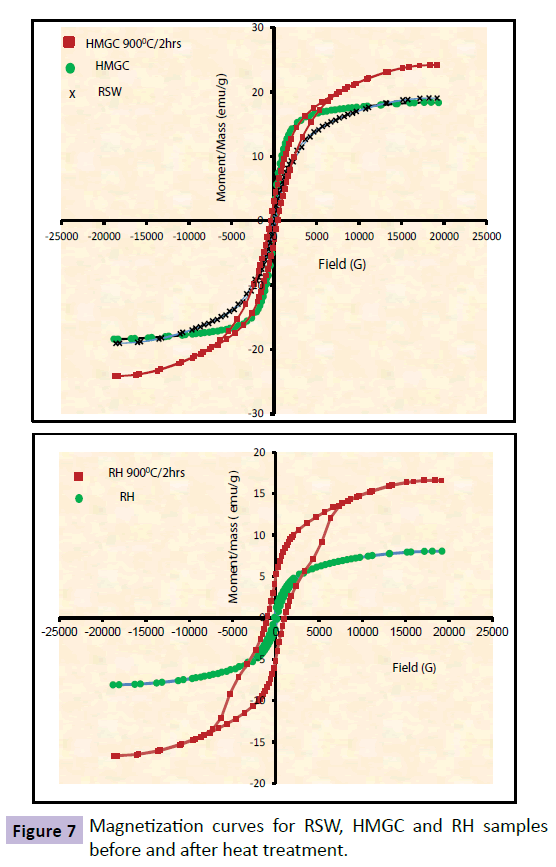
Figures 6 and 7 illustrate the room temperature magnetic hysteresis (M-H) loops of SMGC, RS, HMGC and RH samples before and after heat treatment under a magnetic field strength of ± 20 KOe. Table 3 lists the relevant magnetic parameters; saturation magnetization (Ms), coercivity (Hc), remanence (Mr) obtained from M-H loops. The magnetic field necessary to saturate the samples increased with the increase in the crystallization of Znferrite in soft samples and Ba-hexaferrite in hard magnets. Figure 6 illustrates that Ms increased from 18.393 emu/g for RSW, to 45.175 emu/g for SMGC samples before heat treatment and continued increasing to 66.463 emu/g after heat treatment. In contrast, the magnetization of RS samples declined from 38.678 emu/g to 24.293 emu/g after being exposed to heat treatment at 900°C for 2h. Figure 7 shows that Ms increased from 18.393 emu/g for RSW sample to 19.06 emu/g for HMGC sample and increased to 24.19 emu/g after heat treatment. The same trend is shown for RH samples where Ms increased from 8.0774 emu/g to 16.665 emu/g after heat treatment for 900°C/2 hrs. As expected, saturation magnetization in the samples is shown to be directly proportional to the degree and amount of crystallization of Znferrite and Ba-hexaferrite. It should be noted that Zn-ferrites and Ba-hexaferrite are ferromagnetic materials, whereas hematite is an antiferromagnetic. The results show that hematite and amorphous phases have a very low saturation magnetization. As a consequence, the variation of the saturation magnetization can be attributed to the modification of the quantity of Zn-ferrite and Ba-hexaferrite crystals [65] present in the glass-ceramic samples. Therefore, the maximum magnetization values of 66.463 emu/g for SMGC after heat treatment and 24.19 emu/g for HMGC after heat treatment refer to the heaviest and most intense Zn-ferrite and Ba-hexaferrite crystallization of all the soft and hard magnet samples respectively. The quantity of the magnetic phase present in the glass ceramic samples was determined from the saturation magnetization ratio between the sample and pure magnetite (Ms=72 emu/g) [66]. 92 emu/g is the saturation magnetization of bulk magnetite as reported from the literature [67], O.Bretcanuet. el. Measured the magnetite saturation magnetization as 72 emu/g and they referred this decrease to the powder form of the measured samples. Surface effects can modify the saturation magnetization of a magnetic material, usually lowering the magnetization. The maximum magnetic phases accumulation is 92.4 wt% for heat treated SMGC which is a big increase than what previously reported by other workers [37-39].
| Sample ID | Magnetization (emu/g) | Coercivity(G) | Retentivity(emu/g) |
|---|---|---|---|
| RSW | 18.393 | 106.25 | 1.6785 |
| RS | 38.678 | 16.756 | 0.73946 |
| SMGC | 45.175 | 32.775 | 1.4215 |
| RS 9000C/2hrs | 24.293 | 193.44 | 3.7014 |
| SMGC 9000C/2hrs | 66.463 | 32.966 | 2.3594 |
| RH | 8.0774 | 160.39 | 0.92542 |
| HMGC | 19.06 | 128.77 | 0.95886 |
| RH 9000C/2hrs | 16.665 | 1001.5 | 5.0392 |
| HMGC 9000C/2hrs | 24.19 | 425.32 | 3.1612 |
Table 3 Magnetic properties of RSW, RS, RH, SMGC and HMGC glass ceramics before and after heat treatment.
The coercivity of soft ferrimagnetic glass ceramics varied between 106.25 Oe for the RSW sample and 32.775 Oe for SMGC before heat treatment with a small increase to 32.966 Oe after heat treatment. In HMGC samples heat treatment had a larger effect on coercivity which increased from 106.25 Oe for RSW sample to 128.77 G for the melted HMGC samples before heat treatment and increased to 425.32 G after heat treatment. Similarly, heat treatment had a noticeable effect on the reference samples coericivities with an increase from 16.756 Oe to 193.44 Oe after heat treatment of RS and increased from 160.39 Oe to 1001.5 Oe for RH after heat treatment. The coericivity of a ferrimagnetic material is the intensity of the applied magnetic field required to reduce the magnetization of that material to zero after the magnetization of the sample has been driven to saturation. Coericivity measures the resistance of a ferromagnetic material to become demagnetized. The wide range of coericivity provides for a wide range of applications. Materials with high coericivity, which are called hard ferromagnetic materials, are used to make permanent magnets. Permenant magnets are used in electric motors, magnetic recording media (e.g., hard drives, floppy disks, or magnetic tapes) and magnetic separation. While materials with low coericivity which are said to be soft magnets may be used in microwave devices, magnetic shielding, transformers, or recording heads. The retentivity is found to detect the amount of magnetic materials which can be magnetized [68,69], even in the absence of external magnetic field. In comparison between soft and hard ferrites, Table 3 shows that the retentivity of most of the hard magnets is larger than that of the soft magnets and is related to the area of the hysteresis loops. The area of the hysteresis loops of the hard ferrites is larger than the areas in the case of soft ferrites. The area under the hysteresis loop is proportional to the energy loss and hence the heat generated by a sample under an alternating field and hard ferrites samples are capable of generating more heat. The large variation in the area under the loops for hard and soft ferrites samples provides a controlled heat generation by appropriate choice of sample.
Conclusions
• Two different ferrimagnetic glass ceramics with compositions based on crystallization of Zn-ferrite as soft magnet and Ba-hexaferrite as hard magnet were prepared.
• The influence of the chemical composition, the amount of Zn-ferrite and Ba-hexaferrite crystallization and the microstructure of ferromagnetic glass ceramics on magnetic properties of glass ceramics were investigated.
• The Heat treatment schedule of 900°C for 2 h enhanced the crystallization of Zn-ferrite in soft glass ceramics and Ba-hexaferrite in hard magnetic glass ceramics.
• Nanomagnetic hexaferrite was obtained in the range 24- 34 nm.
• From the magnetic measurements, we conclude that heat treated SMGC had the highest magnetization of 66.5 emu/g and has 92.4 wt% of magnetic phases.
Acknowledgment
This work was supported financially by the Science and Technology Development Fund.
References
- Ramaseshan R, Sundarrajan S, Jose R (2007) Nanostructured ceramics by electrospinning.Journal of applied physics 102:111101.
- Padeletti G, FermoP (2003) Italian Renaissance and Hispano-Moresque lustre-decorated majolicas: imitation cases of Hispano-Moresque style in central Italy. Applied physics A: material science process 77: 125-133.
- Seshadri R (2005) Zinc oxide-based diluted magnetic semiconductors. Current Opinion in Solid State and Material science 9: 1-7.
- YangJH, ZhaoLY, ZhangYJ, WangYX, LiuHL (2008) Paramagnetic behavior of Zn1−xMnxO at room temperature. Cryst Res Technol 43: 999-1003
- Davies MW, KerrisonB, Gross WE, Robson WJ,WichellDF (1970) J Iron Steel Institute 208-348.
- Marghussian VK,Maghsoodipoor A (1999) Fabrication of unglazed floor tiles containing Iranian copper slags. Ceramics International 25:617-622.
- Omar AA, L-Gamal MAE, Kotb W, Mahmoud MM (2000) The Use of Copper Slags for Making Glass-Ceramics. Glass Science and Technology Suppl 73: 51-58.
- Murat M, Martel S, Bouster C (1990) optimization of devitrification processing in the field of glass ceramics example of the devitrification of phosphorous slag. Ceramics International 16: 171-175.
- Boccaccini AR, Bucker M,Bossert M(1996) Glass and glass-ceramics from coal fly-ash and waste glass. Tile and Brick International 12: 515-518
- Boccaccini AR, Bucker M,Bossert J,MarszalekK (1997) Glass matrix composites from coal flyash and waste glass. Waste Management 17: 39-45.
- Boccaccini AR, JanczakJ, Kern HOndracekG (1993) in Proc. 4th.International Symposium on the Reclamation, Treatment and Utilization of Coal Mining Wastes, Vol. II.edited by K. M. Skarzynska, 719
- Cheng TW, Chen YS (2003) On formation of CaO–Al2O3–SiO2 glass–ceramics by vitrification of incinerator fly ash. Chemosphere 51:817-824.
- Cheng TW(2003) Combined glassification of EAF dust and incinerator fly ash. Chemosphere 50: 47-51.
- Pelino M, Karamanov A, PisciellaP, CrisucciS,ZonettiD (2002) Vitrification of electric arc furnace dusts. Waste Management 22: 945-949.
- Morsi MM, KhaterGA, Range KJ (2001) Glass ceramics in the system diopside–anorthite–orthoclase prepared by using some industrial waste materials. Glass Technology 42: 160-164.
- Gorokhovsky, Escalante-Garcia JI, GorokhovskyV,MescheryakovD (2002) Inorganic Wastes in the Manufacture of Glass and Glass-Ceramics: Quartz-Feldspar Waste of Ore Refining, Metallurgical Slag, Limestone Dust, and Phosphorus Slurry. Journal of the American Ceramic Society 85: 285-287.
- Yun YH, Yoon CH, OHJS, Kim SB, Kang BA, et al. (2002) Waste fluorescent glass and shell derived glass-ceramics. Journal of Materials Science 37:3211-3215
- Diaz B, Salgado S, Jordan R, Cruz E, ZayasME (2003) Glass-Ceramics Made from Anodizing Plant Industrial Waste. American Ceramic Society Bulletin 9601-9604.
- PelinoM, CantaliniC, Rincon JMA (1997) Preparation and properties of glass-ceramic materials obtained by recycling goethite industrial waste.Journal of Materials Science 32: 4655-4660.
- Romeo M, Rincon JMA (1999) Surface and Bulk Crystallization of Glass-Ceramic in the Na2O-CaO-ZnO-PbO-Fe2O3-Al2O3-SiO2 System Derived from a Goethite Waste. Journal of the American Ceramic Society 82: 1313-1317.
- MarabiniAM, PlesciaP, MaccariD, BurragatoF, PelinoM (1998) New materials from industrial and mining wastes: glass-ceramics and glass- and rock-wool fiber. International Journal of Mineral Processing 53: 121-134.
- Romeo M, Rincon JMA (1998) Effect of Iron Oxide Content on the Crystallization of a Diopside Glass-Ceramic Glaze. Journal of the European Ceramic Society 22: 883-890.
- KaramanovA, TaglieriG,PelinoM (1999) Iron-Rich Sintered Glass-Ceramics from Industrial Wastes. J Am Ceram Soc 82: 3012-3016.
- Pelino M (2000) Recycling of zinc-hydrometallurgy wastes in glass and glass ceramic materials. Waste Management 20: 561-568.
- PisciellaP, CrisucciS, KaramanovA,PelinoM (2001) Chemical durability of glasses obtained by vitrification of industrial wastes Waste Management 21: 1-9.
- Volodymyr I Shatokha, Oleg OGogenko, Stanislav M Kripak(2011)Utilising of the oiled rolling mills scale in iron ore sintering process.Resources Conservation and recycling 55: 435-440.
- Cho S, LeeJ (2008) Metal Recovery From Stainless Steel Mill Scale by Microwave Heating. Metals and Materials International 14: 193-196
- Martín MI, LópezJ, Jose MTorralba (2012) production of sponge iron powder by reduction of rolling mill scale. iron making& steelmaking 39: 155-162.
- Cho Young-Ki (1991) Making method for ferrite used mill/scales, Patent KR 9103783.
- Osing D (1996) Reuse of metallurgical fines, Patent WO 96/31630.
- Fleischander A, PeslJ,GerbertW (1999) Aspect of recycling of steelworks by-products through the BOF.SEAISI Quarterly 28: 51-60.
- Poulalion A (2001) Process of recycling mill scale of alloyed steel in an electric furnace into a ferro-silicon product, Patent EP 1122319.
- Gaballah NM, ZikryAF, Khalifa MG, FaragAB, El-HussinyNA (2013) Journal of Inorganic Non-Metallic Materials 3: 23-28
- Ebisawa Y,Miyaji F, Kokubo T, Ohura K, NakamuraT (1997) Surface reaction of bioactive and ferrimagnetic glass–ceramics in the system FeO–Fe2O3–CaO–SiO2. J Ceram SocJpn105: 947-951.
- O'HoroM, SteinitzR (1968) Characterization of devitrification of an iron-containing glass by electrical and magnetic properties. Mater Res Bull 3: 117-125.
- AuricP, Dang NV, Bandyopadhyay AK, Zarzycki J (1982) Superparamagnetism and ferrimagnetism of the small particles of magnetite in a silicate matrix. J Non-crystSolids 50:97-106.
- BretcanuO, SprianoS, VerneE, CoissonM, TibertoP, et al. (2005) The influence of crystallized Fe3O4 on the magnetic properties of coprecipitation-derived ferromagneticglass–ceramics. ActaBiomater1: 421-429.
- Abdel-HameedSAM, HessienMM, AzoozMA (2009) Preparation and characterization of some ferromagnetic glass-ceramics contains high quantity of magnetite. Ceram Int 35: 1539-1544.
- Abdel-HameedSAM, Abeer M ElKady (2012) Effect of different additions on the crystallization behavior and magnetic properties of magnetic glass-ceramic in the system Fe2O3-ZnO-CaO-SiO2. J Adv Mater 3: 167-175.
- ShirkBT, BuessemWR (1970) Magnetic properties of barium ferrite formed by crystallization of a glass.J Am Ceram Soc 53: 192-196.
- GornertP, SinnE, SchuppelW, PfeifferH, RoslerM, et al. (1990) Structural and magnetic properties of BaFe12-2x CoxTixO19 powders prepared by the glass crystallization method. IEEE Trans Magn 26: 12-14.
- CetasTC,GrossEJ, ContractorY (1998) A ferrite core/metallic sheath thermoseed for interstitial thermal therapies. IEEETrans Biomed Eng45: 68-77.
- Evans BJ, HafnerSS, WeberHP (1971) Electric field gradients at Fe in ZnFe2O4 and CdFe2O4. J ChemPhys 55: 5282.
- Att-AllahSS, FayekMK (2000) Effect of Cu substitution on conductivity of Ni–Al ferrite. J PhysChem Solids 61: 1529-1534
- MahmoudMH, HamdehHH, HoJC, O'SheaMJ, Walker JC (2000) Mössbauer studies of manganese ferrite fine particles processed by ball-milling.J MagnMagn Mater 220: 139-146.
- MullerR, UlbrichC, SchuppelW, SteinmetzH, SteinbeibE (1999) Preparation and properties of barium-ferrite-containing glass ceramics. J Eur Ceram Soc 19: 1547-1550.
- Lee CK, Speyer RF (1994) Glass formation and crystallization of barium ferrite in theNa2O–BaO–Fe2O3–SiO2 system. J Mater Sci 29: 1348-1351.
- Sohn SB, Choi SY, ShimIB (2002) PreparationofBa–ferrite containing glass–ceramics in BaO– Fe2O3–SiO2. J MagnMagn Mater 293:533-536.
- RezlescuL, RezlescuE, PopaPD, RezlescuN (1999) Fine barium hexaferrite powder prepared by the crystallization of glass. J MagnMagnMater193: 288-290
- GornertP, SinnE, RoslerM (1991) Crystalline materials:Growth and characterization 58:129-148.
- Abdel-Hameed SAM, MarzoukMA, Abdel-GhanyAE (2011) Magnetic properties of nanoparticles glass-ceramic rich with copper ions. J Non-cryst solids 357: 3888-3896
- SafarikovaM, SafarikI (2000) The application of magnetic techniques in biosciences. MagnElectr Sep 10:223-252.
- BabesL, DenizotB, TanguyG, JeuneJJL, JalletP (1999) Synthesis of iron oxide nanoparticles used as MRI contrast agents: a parametric study. J Colloid Interface Sci 212: 474-482.
- SunSH, MurrayCB, WellerD, FolksL, Moser A (2000) MonodisperseFePt nanoparticles and ferromagnetic FePtnanocrystalsuperlattices. Nature 287: 1989-1992.
- El-ShennawiAWA, MorsyAM, SerryMA (1990) Investigation of lithium magnesium-aluminosilicate solid solutions crystallized in some cordierite-zircon ceramics containing lithium. J of MatSci 25: 1429-1434.
- SocoliucV, BicaD, VekasL (2003) Estimation of magnetic particle clustering in magnetic fluids from static magnetization experiments. J. Colloid Interface Sci 264: 141-147
- LuAH, SchmidtW, MatoussevitchN, BönnemannH, SpliethoffB, et al. (2004) Nanoengineering of a magnetically separable hydrogenation catalyst.AngewChemInt Ed 43: 4303-4306.
- Sharifi I, Shokrollahi H, Amiri S (2012) Ferrite-based magnetic nanofluids used in hyperthermia applications. J. MagnMagn Mater 324: 903-915.
- El-Shennawi AWA, MorisMM, KhaterGA, Abdel-HameedSAM (1998) Thermodynamic investigation of crystallization behavior of pyroxenic basalt-based glasses. J Therm Anal 51: 553-560.
- KlugHP, Alexander LE (1974) X-Ray Diffraction procedures for polycrystalline and amorphous materials. Wiley New York.
- Hammond C (1997) The Basic of crystallography and diffraction. Oxford university press, New York.
- ScherrerP, GöttingerNachrichten Gesell. (1918) 2: 98.
- Patterson A (1939)TheScherrer Formula for X-Ray Particle Size Determination.Phys Rev 56: 978–982.
- Myers HP (2002) Introductory Solid State Physics.Taylor & Francis.
- Roy MK, Haldar B, VermaHC (2006) Characteristic length scales of nanosize zinc ferrite. Nanotechnology 17: 232.
- BretcanuO, VerneE, Coisson M, TibertoP,AlliaP (2006) Temperature effect on the magnetic properties of the coprecipitation derived ferromagnetic glass-ceramics. Journal of Magnetism and Magnetic Materials 300: 412-417.
- CullityRD (1972) Introduction of magnetic materials.Addison-Wesley.
- Abdel-HameedSAM, Rawhia LE (2012) Effect of La2O3, CoO,Cr2O3 and MoO3 nucleating agents on crystallization behavior and magnetic properties of ferromagnetic glass-ceramic in the system Fe2O3.CaO.ZnO.SiO2. J Mater Res Bull 47: 1233-1238.
- Abdel-HameedSAM, Ibrahim MHamed, Nehal A. Erfan (2014) Utilization of iron oxide bearing pellets waste for preparing hard and soft ferromagnetic glass ceramics. Journal of advanced ceramics 3: 259-268
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences