Sealing Holes of an Anodic Aluminum Oxide Alloy by Nanotitania for Enhanced Corrosion Resistance
Menglei Chang, Hongyang Wei, Dongchu Chen*, Huawen Hu*, Yuyuan Zhang, Xiufang Ye, Kewei Zeng, Dongmu Li
DOI10.21767/2471-9838.100024
Menglei Chang, Hongyang Wei, Dongchu Chen*, Huawen Hu*, Yuyuan Zhang, Xiufang Ye, Kewei Zeng and Dongmu Li
College of Materials Science and Energy Engineering, Foshan University, Foshan, Guangdong, 528000
- *Corresponding Authors:
- Dr. Dongchu Chen
College of Materials Science and Energy
Engineering, Foshan University
Foshan, Guangdong 528000
Tel: +8613827789893
E-mail: cdcever@163.com
- Dr. Huawen Hu
College of Materials Science and Energy
Engineering, Foshan University
Foshan, Guangdong 528000
Tel: +8613006700167
E-mail: huawenhu@126.com
Received Date: June 21, 2017 Accepted Date: July 01, 2017 Published Date: June 04, 2017
Citation: Chang M, Wei H, Chen D, et al. Sealing Holes of an Anodic Aluminum Oxide Alloy by Nanotitania for Enhanced Corrosion Resistance. Nano Res Appl. 2017, 3:2.doi: 10.21767/2471-9838.100024
Abstract
This study aims to explore an effective, novel, and environmentally friendly method that overcomes the limitations and shortcomings existing in the traditional approaches for sealing holes on aluminum alloy. Herein, the holesealing treatment of an Anodic Aluminum Oxide (AAO) alloy film using two types of titanium sources with a high stability in aqueous electrolytes, that is, ammonium fluorotitanate and titanium potassium oxalate, to electrically deposit nanotitania on the film surface was investigated. The nanotitania deposited electrochemically for hole sealing has the advantages of the high physiochemical stability, low cost, and non-toxicity, which can thus readily improve the corrosion resistance of the sealed AAO in an environmentally friendly manner. Such sealed AAO can also result in a UV-shielding performance due to the commonly-known UV absorption properties of nanotitania. The hole sealing effect is also compared between the two systems involving the two types of titanium sources at different concentrations, voltages and time. The optimization of the preparation conditions is achieved by means of weight loss measurements. Potentiodynamic scan and electrochemical impedance spectroscopy results reveal that the hole-sealed sample-based on ammonium fluorotitanate shows a higher corrosion resistance as compared to the one based on titanium potassium oxalate. Significantly, the optimal conditions for the hole sealing of the aluminum alloy are evidenced to be the concentration of ammonium fluorotitanate of 0.1 mol/L, AC voltage of 3 V, and time of 900 s.
Keywords
Anodic Oxidation of Aluminum Alloy; Electrical Deposition; Hole Sealing; Titania
Introduction
As the second large category of metallic materials, aluminum and its alloys have been used extensively in the research and engineering industries, just following after iron and steel [1-4]. This is because of their light weight, high specific strength, and superior physical and chemical properties [1,2]. As a result, there are various promising applications for them, e.g., in architecture, automobile, and decoration [5-8]. Generally, anodic oxidation is employed for fabrication of such aluminium alloys, which can result in a highly porous oxidation film [9]. Such a film possesses a strong physical adsorption capability and a high chemical reactivity, facilitating the adsorption of air pollutants and hence causing the corrosion of aluminum plates [10,11]. The anodic oxidation film of the aluminum alloys exhibits a honeycombed porous structure, with a rather low average pore diameter. It has also been reported that the number of pores can reach as high as 7.7 mm-2 × 108 mm-2. Therefore, the hole filling technology is urgently needed to enhance the corrosion resistance, antipollution and electrical insulating properties, keeping the surface of the aluminium alloys in a good state [12].
Among the commonly-used methods for sealing the holes of the oxidation film of aluminum alloys, including hydrothermal and metal-salt solution sealing, etc. [13-16], hydrothermal sealing is the most commonly-used one due to its good sealing effect that can also be easily achieved by placing the porous oxidation film into hot water (80°C~100°C) [14,16]. Therefore, this hydrothermal sealing method possesses the advantages of facile operation and high efficiency. On the other hand, there also exist some demerits including high energy consumption, a long time for hole sealing, degraded film hardness, high sealing temperature, uncomfortableness for operators, and corrosions as caused by volatized water vapor [17]. Sealing by using nickel salt solutions can effectively improve the sealing quality, lower the sealing temperature, reduce the sealing time, and stabilize the organic dye molecules in the micropores of the anodic oxidation film, but the insurmountable problem lies in the environmental pollution as caused by nickel ions [18]. Additionally, sealing with a bichromate solution has the merits of easy operation and satisfactory corrosion resistance, which can thus be applied for protection applications through sealing the holes of the anodic aluminum oxide (AAO) film on aluminum alloys, but the high toxicity of hexavalent chromium, even with a carcinogenic effect, largely limits the practical applications [13].
Nanotitania has been regarded as the most promising semiconductor catalyst and environmentally friendly photocatalytic material, owing to its impressive photocatalysis, hydrophilicity, high electron-hole counter potential, and good physiochemical stability, low cost, non-toxicity and ready availability [19-24]. As a consequence, nanotitania has been extensively explored across various scientific disciplines [ 21,22,25-41]. For instance, nanotitania can be used to seal aluminum oxide for corrosion resistance and UV-protection by virtue of the commonly-known physiochemical stability and UVshielding performance of the nanotitania [42,43]. However, it remains a great challenge to use nanotitania as a hole sealing material for AAO film [23-34] This study aims to grow a layer of nanotitania film for the purpose of sealing the holes of the AAO film on the aluminum alloy.
A large number of methods have been employed to prepare the titania film primarily based on the vapor phase, liquid phase, solgel, precipitation, and electrical deposition [21,22,25-43]. In this study, an anodic oxidation method was adopted for deposition of nanotitania under alternating voltage conditions, leading to the generation of a composite film. Our method has the following advantages: (i) due to the electrical deposition proceeded in a solution at a low temperature, no remaining thermal stress exists within the composite film, resulting in enhanced adhesion interactions between the substrate and composite film, (ii) because of the linear process of the electrical deposition, the film can be uniformly grown on the aluminum oxide matrix, with complex shape and porous surface, for hole sealing, and (iii) by means of modulating current, voltage, and pH, temperature and concentration of the solution, the thickness, chemical composition and porosity can be finely controlled, and (iv) low invest for setting the electrical deposition equipment, high utilization efficiency of raw materials, low cost during the production, ease of manufacturing, and facile operation. Therefore, such a technique holds a great promise for widespread applications. In addition, the electrical deposition approach exhibits cost-effective and simple to manufacturing characteristics, which takes only a short time to load a large amount of titania on the aluminum alloy in one cycle, outperforming the multiple coating process of sol-gel techniques and hence showing more promising applications of coating titania on the iron and steel surface. Consequently, broad prospects of practical applications can be expected.
Materials and Methods
Aluminum oxide preparation
An aluminum alloy (6063) plate with a size of 75 mm2 × 50 mm2 was cleaned by ultrasonication with a small amount of detergent for 3 min to 5 min, so as to remove the oily contaminants from the plate surface. Afterwards, deionized (DI) water was used to thoroughly wash the plate, followed by washing with an alkaline solution for 3 min to 5 min and then with DI water. After these washings, the plate was placed into the pre-prepared polishing solution for 2 min to 3 min of polishing treatment, followed by thoroughly washing. After that, the plate was put into an electrolytic cell with a sulfuric acid electrolyte (17%). The aluminium alloy and lead plates were used as anode and cathode respectively, with a distance of approximately 20 cm. The oxidation processing was then carried out at a stable voltage of 3.7 V for 2000 s.
Hole sealing by two types of titanium sources
Selecting titanium potassium oxalate and ammonium hexafluorotitanate as the precursor of nanotitania is by considering their higher stability in aqueous electrolytes as compared to other kinds of precursors such as Tetraisopropyl Titanate (TIPT) and TiCl4 due to the ready hydrolysis properties of TIPT and TiCl4 [44,45].
The hole sealing achieved by titanium potassium oxalatebased electrical deposition is described as follows: a preprepared solution containing titanium potassium oxalate with a concentration of 0.01 mol/L, 0.05 mol/L or 0.1 mol/L was adopted as the electrical deposition liquid. After anodic oxidation, the aluminum alloy was dipped into the mixed electrical deposition liquid for 900 s. Upon connection of the circuit, the aluminum alloy and lead plates became the cathode and anode, respectively. The alternating voltage was set to be 3 V, 3.5 V or 4 V, with the deposition time as 300 s, 900 s or 1800 s. The optimized parameters were obtained by weight loss experiments on the samples being prepared under the conditions of different concentrations of deposition liquid, voltages, and time.
The procedures for the hole sealing by ammonium hexafluorotitanate-based electrical deposition was given below. The pre-prepared ammonium hexafluorotitanate solutions with concentrations of 0.01 mol/L, 0.05 mol/L, 0.1 mol/L were used as the electrical deposition liquid. The anodically oxidized aluminum alloy was dipped into the prepared electrical deposition liquid for 900 s. After the circuit connection, the aluminum alloy and lead plates were employed as the cathode and anode respectively. The alternating voltage was to be 3, 3.5, and 4 V, with the deposition time as 300 s, 900 s or 1800 s. The optimized parameters were found out by weight loss experiments on the samples being prepared under conditions of different concentrations of deposition liquid, voltages, and time.
Electrochemical measurements of the samples after the hole sealing:
Potential scan in 3.5% NaCl acidic (pH 2.0), neutral (pH 7.0), and alkaline (pH 12) environments was performed for the samples prepared under optimized conditions in the weight loss experiment. The scanning range, speed and testing time were set as -1.5 V~1.5 V, 20.0 mV/s, and 5 min, respectively. The electrochemical polarization curves were obtained on an electrochemical workstation (CS-310, Wuhan Branch, Instrument Co., Ltd.) using a three-electrode system. Pt and saturated calomel electrodes were used as the counter and reference electrodes, respectively. The exposure area of the specimen was 5.7 cm2, with the other location sealed by epoxy. According to the above optimized result, AC impedance measurement was performed on the optimized sample. The parameters used for the measurement were given as follows:
(i) field grounding mode;
(ii) testing frequency range of 10-2 Hz~105 Hz;
(iii) AC amplitude of sine wave of 10 mV;
(iv) 470 pF and 2.2 nF broad band responses at the frequencies of >10 and <10, respectively; and
(v) filter set as 470 nF.
The fitting processing of the obtained AC impedance data was performed by a ZView impedance analysis software, and then by an Origin software to convert the data to figures.
Results and Discussion
Weight loss experiments on the system holesealed by using two types of titanium sources as the precursor of nanotitania
For the same deposition liquid formulation used for hole sealing, the parameters of time, voltage, and concentration become important parameters (Tables 1 and 2). Through the comparison of the weight loss testing data (the weight loss of smaller than 30 mg/mm2 can be considered to be qualified), it can be noted that the weight loss for hole sealing is increased with increasing the voltage. The longer the time, the lower the weight loss for hole sealing. In addition, increasing concentration lowers the weight loss for hole sealing. On the other hand, for the titanium potassium oxalate as the hole sealing liquid, the weight loss is initially increased and then decreased, with increasing the concentration. Concerning the ammonium fluorotitanate as the hole sealing liquid, the sample 8 presents a minimum weight loss of 3.2 mg/mm2, while the minimum weight loss become 8.3 mg/mm2 for the sample 6 in the case of the titanium potassium oxalate as the hole sealing liquid. The lower weight loss for hole sealing indicates the higher corrosion resistance and hence the better hole sealing effect. As a consequence, the optimized conditions for electrical deposition of titania are presented in the following Table 3.
| Parameter | A Conc./mol/L | B Time/s | C Voltage/V | Weight loss Δm/(mg/mm2) |
|---|---|---|---|---|
| Testing No. | ||||
| 1 | 0.01 | 300 | 3 | 11.2 |
| 2 | 0.01 | 900 | 3.5 | 35.3 |
| 3 | 0.01 | 1800 | 4 | 26.2 |
| 4 | 0.05 | 300 | 3.5 | 19.2 |
| 5 | 0.05 | 900 | 4 | 45.3 |
| 6 | 0.05 | 1800 | 3 | 8.3 |
| 7 | 0.1 | 300 | 4 | 57.5 |
| 8 | 0.1 | 900 | 3 | 24.1 |
| 9 | 0.1 | 1800 | 3.5 | 38.4 |
Table 1: The weight loss results of titanium potassium oxalate-based electrodeposition of Nanotitania as a function of deposition conditions including the concentration, time and voltage.
| Parameter | A Conc./mol/L | B Time/s | C Voltage/V | Weight loss Δm/(mg/mm2) |
|---|---|---|---|---|
| Testing No. | ||||
| 1 | 0.01 | 300 | 3 | 18.4 |
| 2 | 0.01 | 900 | 3.5 | 44.8 |
| 3 | 0.01 | 1800 | 4 | 63.9 |
| 4 | 0.05 | 300 | 3.5 | 21.7 |
| 5 | 0.05 | 900 | 4 | 19.3 |
| 6 | 0.05 | 1800 | 3 | 9.7 |
| 7 | 0.1 | 300 | 4 | 14.6 |
| 8 | 0.1 | 900 | 3 | 3.2 |
| 9 | 0.1 | 1800 | 3.5 | 6.4 |
Table 2: The weight loss results of ammonium fluoride titanate-based electrodeposition of nanotitania as a function of deposition conditions including the concentration, time and voltage.
| pH=2.0 | Electrochemical parameter | Polarization resistance/Ω | |||
|---|---|---|---|---|---|
| Ecorr/V | Icorr/A | Bc/mV | Ba/mV | ||
| Ammonium fluorotitanate system | -0.1673 | 1.1825 × 10-7 | 488.3 | 1186 | 3.047 × 106 |
| Titanium potassium oxalate system | -0.5566 | 1.0771 × 10-6 | 388.6 | 600 | 4.446 × 105 |
Table 3: Optimization of the conditions for electrodeposition of titanium dioxide.
Polarization curve measurements for the two types of titanium sources-sealed systems:
Based on the above-optimized results as obtained from weight loss experiments, the experimental conditions are given as follows:
(i) Deposition voltage of 3 V;
(ii) Concentration of ammonium fluorotitanate of 0.10 mol/L;
(iii) Deposition time of 900 s;
(iv) Concentration of titanium potassium oxalate of 0.05 mol/L; and
(v) Deposition time of 1800 s.
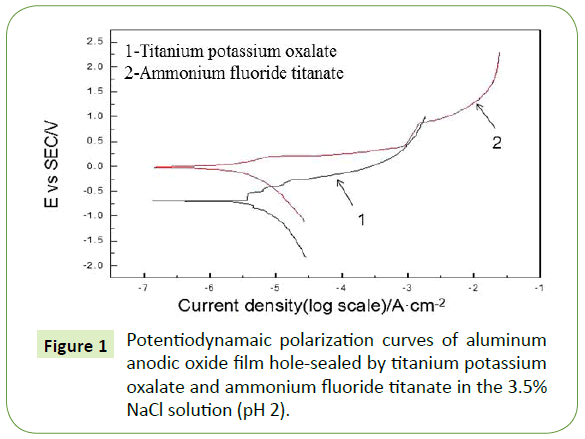
Under the above-detailed conditions, polarization curves are measured. As for the system with the 3.5% NaCl solution (pH 2.0), CS-310 electrochemical workstation was adopted for the obtaining the polarization curve, with the scanning range of the anodic polarization curve of -1.5 V~1.5 V, scanning speed of 20.0 mV/s, and detection period of 5 min. The resulting polar curves are presented in Figure 1.
Concerning the system with the 3.5% NaCl solution (pH 2.0), selfcorrosion potential for hole sealing by ammonium fluorotitanate is higher than that by titanium potassium oxalate, revealing the lower tendency of corrosion (Table 4). The latter exhibits a higher self-corrosion current density as compared to the former, which also indicates a higher corrosion speed leading to easier corrosion. The value of polar resistance can be calculated according to the polar resistance equations. The higher the polar resistance, the higher the quality of the hole sealing. The corrosion becomes more difficult to take place. These results can give us a conclusion that the AAO film (as sealed using amonium fuorotitanate as the titanium source) exhibits higher corrosion resistance as compared to the one sealed with titanium potassium oxalate as the titanium source.
| pH=2.0 | Electrochemical parameter | Polarization resistance/Ω | |||
|---|---|---|---|---|---|
| Ecorr/V | Icorr/A | Bc/mV | Ba/mV | ||
| Ammonium fluorotitanate system | -0.1673 | 1.1825 × 10-7 | 488.3 | 1186 | 3.047 × 106 |
| Titanium potassium oxalate system | -0.5566 | 1.0771 × 10-6 | 388.6 | 600 | 4.446 × 105 |
Table 4: Electrochemical parameters adopted for obtaining potentiodynamaic polarization curves at pH 2.
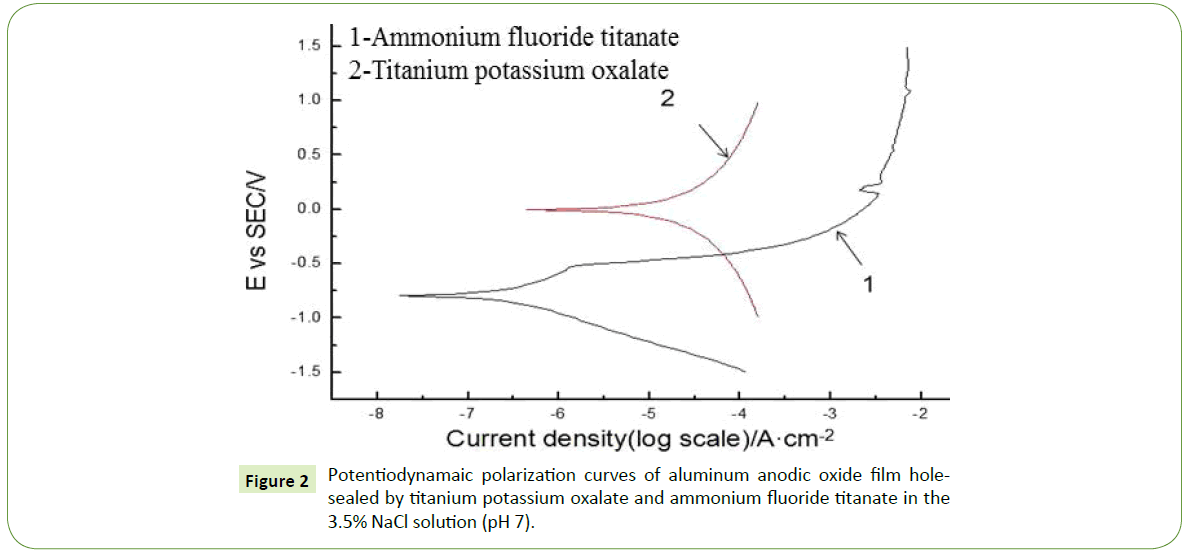
As for the system with the 3.5% NaCl solution (pH 7), CS-310 electrochemical workstation was used to measure the polar carvers, with the electrokinetic potential scanning range of -1.5 V~1.5 V, speed of 20.0 mV/s and time of 5 min. The obtained polar curves are presented in the following Figure 2.
In the 3.5% NaCl solution system, the AAO film with the holes sealed with the titanium potassium oxalate as the sealing precursor shows a higher self-corrosion potential than the ammonium fluorotitanate-sealed one, meaning the higher tendency of corrosion (Table 5). The former shows a higher self-corrosion current density as compared to the latter, also indicating the easier corrosion with a higher corrosion speed. According to the polar resistance Eq., the polar resistance can be calculated. The larger the polar resistance, the better the quality of the AAO film with the holes sealed. The smaller tendency of corrosion can thus be achieved.
| pH=7.0 | Electrochemical parameter | Polar resistance/Ω | |||
|---|---|---|---|---|---|
| Ecorr//V | Icorr/A | Bc/mV | Ba/mV | ||
| Ammonium fluorotitanate system | -0.9332 | 5.1786 × 10-6 | 222.1 | 359.08 | 4.8659 × 106 |
| Titanium potassium oxalate system | -0.0202 | 9.7656 × 10-6 | 614.9 | 659.3 | 4.0516 × 106 |
Table 5: Electrochemical parameters of potentiodynamaic polarization curves at pH 7.
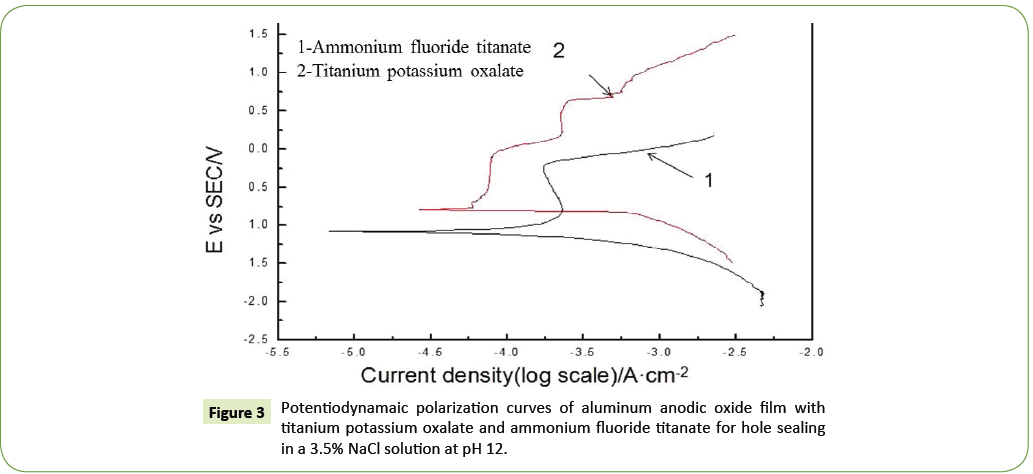
In the 3.5% NaCl solution system (pH 12), CS-310 electrochemical workstation was used to measure the polar carvers, with the electrokinetic potential scanning range of -1.5 V~1.5 V, speed of 20.0 mV/s and time of 5 min. The obtained polar curves are presented in the following Figure 3.
In the system with a 3.5% NaCl solution (pH 12), the AAO film with the holes sealed using ammonium fluorotitanate as the precursor shows an apparently lower self-corrosion potential as compared to the titanium potassium oxalate-sealed one, indicating a lower tendency of corrosion (Table 6). The latter also exhibits a higher self-corrosion current density, also revealing a higher corrosion speed and hence easier corrosion. The polar resistance can be calculated according to the polarization resistance equations. The larger value of polar resistance indicates the higher quality of the sealed film and hence higher resistance to the corrosion.
| pH=12 | Electrochemical parameter | Polarization Resistance/Ω | |||
|---|---|---|---|---|---|
| Ecorr/V | Icorr/A | Bc/mV | Ba/mV | ||
| Ammonium fluorotitanate system | -0.7385 | 6.60093 × 10-4 | 513.3 | 770.6 | 1.9874 × 103 |
| Titanium potassium oxalate system | -0.1.086 | 2.4595 × 10-7 | 649.4 | 1245.8 | 1.7907 × 107 |
Table 6: Electrochemical parameters of Potentiodynamaic polarization curves at pH 12.
From the results presented in Figures 1-3, it can be noted that, for all the systems with the NaCl solution under acidic to alkaline conditions, AAO film sealed with ammonium fluorotitanate as the precursor shows lower self-corrosion potentials as compared to the titanium potassium oxalate-sealed one, thus indicating their lower corrosion speeds. The polar resistance as calculated from the polar resistance equations also reveals the higher quality of the sealed AAO film using ammonium fluorotitanate as the precursor. These findings can be attributed to the stronger chemical bonding between Al2O3 and TiO2 as formed by AC deposition with ammonium fluorotitanate. Moreover, the layers of the film are more compact and stable.
AC impedance measurements for the system hole-sealed by two types of titanium sources
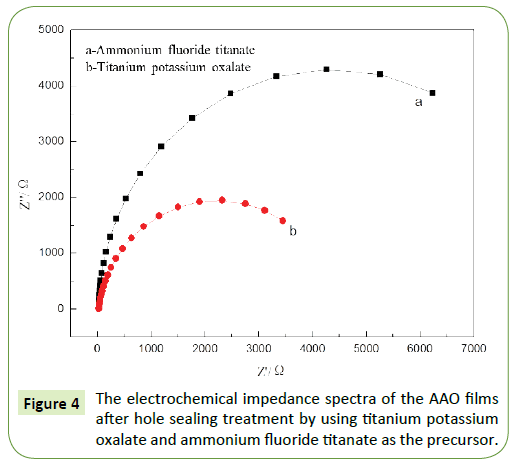
On the basis of the above optimized results, it can be found that the best hole sealing effect can be achieved at 3 V; consequently, the samples sealed at 3 V (using both ammonium fluorotitanate and titanium potassium oxalate as the precursor) were used to conduct the impedance measurements, with the results shown in Figure 4.
Under the same conditions, the electrochemical impedance spectra are captured for the ammonium fluorotitanate- and titanium potassium oxalate-sealed samples at 3 V. Based on the analysis results of the electrochemical impedance spectra, the larger the radius of capacity reactance arc, the higher is the impedance, along with a higher resistance and hence a higher corrosion resistance (Figure 4). It can also be found that the radius of the impedance pattern for ammonium fluorotitanatesealed sample is obviously larger than that for titanium potassium oxalate-sealed sample, which is an indication of the more compact and stable AAO film with the holes sealed using ammonium fluorotitanate as the precursor, together with higher impedance. This results in the higher corrosion resistance as compared to the titanium potassium oxalate-sealed sample.
Conclusion
By virtue of the weight loss experiment, this study has demonstrated that the minimum value of weight loss reaches 3.2 mg/mm2 as for the system sealed by the nanotitania using ammonium fluorotitanate as the precursor for electrical deposition-based hole sealing, while the corresponding minimum value is as high as 8.3 mg/mm2 as for the system involving titanium potassium oxalate. Both the titanium sources can contribute to the hole sealing and meet with the demand for corrosion resistance of the anodic aluminum oxide. More compact TiO2 film are formed on the surface of the aluminum alloy as sealed by the nanotitania with ammonium fluorotitanate as the precursor, as indicated by the potential scanning and AC impedance measurements after the optimization from the weight loss experiment. This contributes to the higher stability and corrosion resistance as compared to the titanium potassium oxalate-sealed one under all the pH conditions considered in this study. The optimal condition for hole sealing is demonstrated to be ammonium fluorotitanate of 0.1 mol/L, AC voltage of 3 V, and deposition time of 900 s.
Acknowledgements
We greatly appreciate the Engineering Project for Innovating and for Enhancing Universities of Guangdong Province (2016GCZX008), Natural Science Foundation of China (21671038), the Project Funded by Engineering Technology Center of Foshan City (2014GA000355), the Special Funds for Innovation of Science and Technology of Foshan City (2014AG10009), and the Key Platform Financing Programs from the Education Department of Guangdong Province (gg041002).
References
- Modaresi R, Pauliuk S, Løvik AN, Müller DB (2014) Global carbon benefits of material substitution in passenger cars until 2050 and the impact on the steel and aluminum industries. Environ Sci Technol 48: 10776-10784.
- Quazi MM, Fazal MA, Haseeb ASMA, Yusof F, Masjuki HH, et al. (2015) Laser-based surface modifications of aluminum and its alloys. Crit Rev Solid State Mater Sci 41: 106-131.
- Xhanari K, Finšgar M (2016) Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solutions: A review. Arab J Chem
- Chen W, Yin X, Ma D (2014) A bottom-up analysis of China’s iron and steel industrial energy consumption and CO2 emissions. Applied Energy 136: 1174-1183.
- RodiÃÆââ¬Å¾ÃâàP, Milošev I (2015) Electrochemical and salt spray testing of hybrid coatings based on Si and Zr deposited on aluminum and its alloys. J Electrochem Soc. 162: C592-C600.
- Abdallah M, Kamar EM, El-Etre AY, Eid S (2016) Gelatin as corrosion inhibitor for aluminum and aluminum silicon alloys in sodium hydroxide solutions. Protection of metals and physical chemistry of surfaces. 52: 140-148.
- Santos MC, Machado AR, Sales WF, Barrozo MAS, Ezugwu EO (2016) Machining of aluminum alloys: a review. Int J Adv Manuf Technol 86: 3067-3080.
- Wei H, Hu H, Chang M, Zhang Y, Chen D, et al. (2017) Improving solar thermal absorption of an anodic aluminum oxide-based photonic crystal with Cu-Ni composite nanoparticles. Ceram Int.
- Fomin A, Dorozhkin S, Fomina M, Koshuro V, Rodionov I, et al. (2016) Composition, structure and mechanical properties of the titanium surface after induction heat treatment followed by modification with hydroxyapatite nanoparticles. Ceram Int 42: 10838-10846.
- Trellu C, Péchaud Y, Oturan N, Mousset E, Huguenot D, et al. (2016) Comparative study on the removal of humic acids from drinking water by anodic oxidation and electro-Fenton processes: Mineralization efficiency and modelling. Appl Catal B 194: 32-41.
- Santos MC, Elabd YA, Jing Y, Chaplin BP, Fang L (2016) Highly porous Ti4O7 reactive electrochemical water filtration membranes fabricated via electrospinning/electrospraying. Aiche J 62: 508-524.
- Toptan F, Alves AC, Pinto AMP, Ponthiaux P (2017) Tribocorrosion behavior of bio-functionalized highly porous titanium. Journal of the Mechanical Behavior of Biomedical Materials 69: 144-152.
- Shang Y (2016) The effects of different sealing techniques for anodic film of Al-12.7Si-0.7 Mg alloys. Int J Electrochem Sci 11: 5234-5244.
- Dey A, Rani RU, Thota HK, Sharma AK, Bandyopadhyay P, et al. (2013) Microstructural, corrosion and nanomechanical behaviour of ceramic coatings developed on magnesium AZ31 alloy by micro arc oxidation. Ceram Int 39: 3313-3320.
- Chai Y, Zhou X, Zhang H (2017) Effect of oxidation treatment on KD–II SiC fiber–reinforced SiC composites. Ceram Int 43: 9934-9940.
- Pusch R, Ramqvist G, Knutsson S (2016) Modern method for sealing deep boreholes. Engineering Geology 202: 132-142.
- Wang S, Peng H, Shao Z, Zhao Q, Du N (2016) Sealing of anodized aluminum with phytic acid solution. Surf Coat Technol 286: 155-164.
- Kim M, Yoo H, Choi J (2017) Non-nickel-based sealing of anodic porous aluminum oxide in NaAlO2. Surf Coat Technol 310: 106-112.
- Xiong Z, Zhao XS (2013) Titanate@TiO2 core–shell nanobelts with an enhanced photocatalytic activity. J Mater Chem A 1: 7738.
- Hamadanian M, Sayahi H, Zolfaghari A, Jabbari V (2015) The modified electrode position of uniform and defect-free TiO2 nanolayer onto stainless steel substrate with enhanced photocatalytic performance. Nano Res Appl 1: 1-5.
- Wang X, Hu H, Yang Z, Kong Y, Fei B, et al. (2015) Visible light-active sub-5 nm anatase TiO2 for photocatalytic organic pollutant degradation in water and air and for bacterial disinfection. Catal Commun 72: 81-85.
- Hu H, Chang M, Wang X, Chen D (2017) Cotton fabric-based facile solar photocatalytic purification of simulated real dye wastes. J Mater Sci 52: 9922-9930.
- Jeong C, Lee J, Sheppard K, Choi CH (2015) Air-impregnated nanoporous anodic aluminum oxide layers for enhancing the corrosion resistance of aluminum. Langmuir 31: 11040-11050.
- Hu N, Dong X, He X, Browning JF, Schaefer DW (2015) Effect of sealing on the morphology of anodized aluminum oxide. Corros Sci 97: 17-24.
- Hu HW, Xin JH, Hu H (2013) Highly efficient graphene-based ternary composite catalyst with polydopamine layer and copper nanoparticles. Chem Plus Chem 78: 1483-1490.
- Hu HW, Chen GH, Fang M, Zhao WF (2009) Modification of graphite oxide nanoparticles prepared via electrochemically oxidizing method. Synth Met 159: 1505-1507.
- Hu H, Xin JH, Hu H, Wang X, Miao D, et al. Synthesis and stabilization of metal nanocatalysts for reduction reactions- A review. J Mater Chem A 3: 11157-11182.
- Hu H, Xin JH, Hu H, Wang X, Kong Y (2015) Metal-free graphene-based catalyst—Insight into the catalytic activity: A short review. Appl Catal A Gen 492: 1-9.
- Hu H, Xin JH, Hu H, Wang X (2015) Structural and mechanistic understanding of an active and durable graphene carbocatalyst for reduction of 4-nitrophenol at room temperature. Nano Res 8: 3992-4006.
- Hu H, Xin JH, Hu H, Chan A, He L (2013) Glutaraldehyde–chitosan and poly (vinyl alcohol) blends and fluorescence of their nano-silica composite films. Carbohydr Polym 91: 305-313.
- Hu H, Xin JH, Hu H (2014) PAM/graphene/Ag ternary hydrogel: Synthesis characterization and catalytic application. J Mater Chem A 2: 11319.
- Hu H, Xin J, Hu H, Wang X, Lu X (2014) Organic Liquids-Responsive β-Cyclodextrin-functionalized graphene-based fluorescence probe: Label-free selective detection of tetrahydrofuran. Molecules 19: 7459-7479.
- Hu H, Wang X, Miao D, Wang Y, Lai C, et al. (2015) A pH-mediated enhancement of the graphene carbocatalyst activity for the reduction of 4-nitrophenol. Chem Commun 51: 16699-16702.
- Hu H, Wang X, Lee KI, Ma K, Xin JH (2016) Graphene oxide-enhanced sol-gel transition sensitivity and drug release performance of an amphiphilic copolymer-based nanocomposite. Sci Rep 6: 31815.
- Hu H, Chen L, Chen G (2011) Reinforcement of epoxy with graphite oxide nanoparticles prepared via electrochemical route. Mater Manuf Processes 26: 618-622.
- Hu H, Chen G (2013) Electrochemically modified graphite nanosheets and their nanocomposite films with poly (vinyl alcohol). Polym Compos 31: 1770-1775.
- Hu H, Allan CCK, Li J, Kong Y, Wang X, et al. (2014) Multifunctional organically modified graphene with super-hydrophobicity. Nano Res 7: 418-433.
- Zhang Y, Hu H, Chang M, Chen D, Zhang M, et al. (2017) Non-uniform doping outperforms uniform doping for enhancing the photocatalytic efficiency of Au-doped TiO 2 nanotubes in organic dye degradation. Ceram Int 43: 9053-9059.
- Huo J, Hu H, Zhang M, Hu X, Chen M, et al. (2017) A mini review of the synthesis of poly-123-triazole-based functional materials. RSC Adv 7: 2281-2287.
- Hu H, Chang M, Zhang M, Wang X, Chen D (2017) A new insight into PAM/graphene-based adsorption of water-soluble aromatic pollutants. J Mater Sci 52: 8650-8664.
- Chang M, Hu H, Zhang Y, Chen D, Wu L, et al. (2017) Improving visible light-absorptivity and photoelectric conversion efficiency of a TiO2 nanotube anode film by sensitization with Bi2O3 nanoparticles. Nanomaterials 7: 104.
- Jaroenworaluck A, Pijarn N, Kosachan N, Stevens R (2012) Nanocomposite TiO2–SiO2 gel for UV absorption. Chem Eng J 181: 45-55.
- Tsuzuki T, Wang X (2010) Nanoparticle coatings for UV protective textiles. Research Journal of Textile and Apparel (RJTA) 14: 9-20.
- Yildizhan MM, Sturm S, Gulgun MA (2016) Structural and electronic modifications on TiO2 anatase by Li K or Nb doping below and above the solubility limit. J Mater Sci 51: 5912-5923.
- Zhang P, Zhang C, Xie A, Li C, Song J, et al. (2015) Novel template-free synthesis of hollow@porous TiO2 superior anode materials for lithium ion battery. J Mater Sci 51: 3448-3453.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences