Superparamagnetic Nanoparticles Coated with Various Electric Charges and Structural Changes in α-Synuclein and β-Amyloid Proteins
Ahmadianpour V, Javdani N and Raheb J
1Department of Basic Sciences, Islamic Azad University Science and Research Branch, Tehran, Iran
2Department of Molecular Medicine, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
- *Corresponding Author:
- Raheb J
Department of Molecular Medicine
National Institute of Genetic Engineering and Biotechnology
Tehran, Iran
Tel: +9844787387
E-mail: jam@nigeb.ac.ir
Received Date: November 02, 2019; Accepted Date: November 18, 2019; Published Date: November 26, 2019
Citation: Ahmadianpour V, Javdani N, Raheb J (2019) Superparamagnetic Nanoparticles Coated with Various Electric Charges and Structural Changes in α-Synuclein and β-Amyloid Proteins. Nano Res Appl Vol.5 No.2:1
DOI: 10.36648/2471-9838.5.1.41
Abstract
Alzheimer's and Parkinson's are the most common amyloid diseases in the world that can affect more than 30 million people worldwide. The main cause of this disease is the production of amyloid fibrils, which play an important role in the development of neurological diseases. In fact, the formation of these compounds initiates the fibrillation process in the brain. The two peptides derived from fibrils are β-amyloid and α- synuclein, which form plaques in the brain. Studies have shown that magnetic iron oxide superconducting nanoparticles (SPIONs) that contain iron oxide nuclei affect protein fibrillation. Therefore, in this study, we tested commercial SPION nanoparticles with different electric charges including positive (NH2) negative (COOH) and neutral (Plain) at different concentrations of 50, 25 and 100 g/ml of nanoparticles on fibril kinetics of proteins. We examined this by modifying the fluorescence emission of thioflavin T. The results showed that while lower concentrations of SPION (25 g/ml) caused immediate inhibition of fibrillation, higher concentrations (100 g/ml) increased the rate of fibrillation process. Considering the nanoparticle charge, it was observed that neutral-charged SPIONs and the highest concentration (100 g/ml) were able to speed up the fibrillation process significantly compared to negative or positive SPIONs. The amount of concentration is same. The difference between the fluorescence emission from the β-amyloid fibrillation process and the α-nucleoside-induced fibrillation with different applied loads and concentrations were statistically significant at p<0.05, which indicates that in addition to the presence of nanoparticles, The electrical charge that affects the monomeric proteins in the environment (in the nucleation phase) also affects the concentration of the nanoparticles on the protein compounds. Differences in fluorescence emission were also examined in each of the two types of proteins studied.
Keywords
SPIONs; α-Synuclein; β-amyloid; Fibrillation; Alzheimer's disease; Parkinson's disease
Introduction
Protein gatherings inside and outside the cell are within the central nervous system that lead to symptoms of nervous illnesses such as Parkinson (PD) and Alzheimer's (AD). The insoluble concentrations inside the cell contain the α-synuclein protein, the bodies of Louis, which are found in the brain, cerebellum, hypothalamus, and the automated nervous system of patients with Parkinson's patients, extracellular aggregate of β-peptides that resulted from the abnormal decomposition of Amyloid progenitor protein, and thus the inside the cell of the neuro-fibrillation protein in the hippocampus, the cerebellum, and the cerebral cortex, healthy individuals and elderly Alzheimer's patients, and other mental dementia [1]. Studies have proven that the formation of a protein -based protein -intensive gathering, particularly popular gatherings, can be the main cause of some diseases nervous system such as Alzheimer ’ s disease, Huntington ’ s disease and Parkinson’s disease.
In these diseases, large amounts of protein are widely used outside and inside the cells, where protein plaque is called protein plates, and in which the structure of proteins varies in form of regular β-pages, which varies with their natural structure. Currently more than 20 types of disease -related diseases are known, including Alzheimer's (AD), Parkinson (PD), Huntington (HD), and so on. In these diseases, the complexity of the protein leads to the formation of Amyloids structures that are insoluble and soluble in cell. Infact, the monomers are first bound together to produce oligomers and are then developed through the accumulation of oligomers (insoluble) structures.
The compounds of enriched Amyloids are at β-plates and have 10-20 nm sizes [2]. As mentioned in the introduction section, the mechanism of Amyloid formation is dependent on the nuclease phase, and consists of a slow phase called lag phase, which are associated with each other [3]. This phase, which determines the extent of the process, is looking for the elongation phase where Fibrils are formed by adding quickly to the sub-units. Amyloids are of two types: those who are composed of unfolded proteins such as β-Amyloid and Alfa C nuclein, formed and proteins such as albumin, β-2 and terestiretin (TTR), each of which forms the Amyloid structure depending on the type of amino acids and environmental conditions [4]. The prevalence of Alzheimer's in the age of 60 is about 5-5% higher, but dramatically, it is about 40-40% higher than at the age of 85. Amyloid β-is an amphipathic polypeptide or a molecular weight of 4 kDa, which consists of 42-1 amino acid, and the folding of itself plays an important role in the initiation and progress of Alzheimer's disease. the form of Β-Amyloid Monomer are highly charged in solution, non -structural under the residual unit of β-Amyloid, however, the rest of the sequence consists of two sides of the hydrophobic (16-21) and (30-30) residues, which are believed to play an important role in the oligomerization and are separated by an area containing two amino acids (Glu 22 and asp 23) and a play -unit (Lys 28). It has been suggested that the hydrophobic core at the time of formation Fibrils plays a key role in the accumulation of β-Amyloid. The ventricular fibrillation of 42 is a two -step response. A slow, abrupt period that causes thermodynamic effect to the nuclease stage or (nucleation) is converted to "seed" phase, followed by a rapid process of fibrils and rapid gathering. α-Synuclein is the most common brain protein containing 140 amino acids, including 7 axons. It contains amino acid cystein and tryptophan and is found in pre -synaptic terminals in solution form and to the membrane. It is estimated that α-Synuclein accounts for 1% of the total proteins in the cytosol region of the brain solution. This protein is first characterized by Mahmoudi et al. [5] as neural cellular protein, which is located in the pre -synaptic terminals of the nucleus and named "c-nuclein". It was found that there is a connection between the mutation in the α- Synuclein and the Parkinson’s disease (PD), and the collection of this protein forms the main compound of Lewy, characterized by Parkinson’s disease. Some researchers believe that pharmaceutical companies and their research departments, using these studies and hypotheses, are seriously starting to look for medications to prevent Aβ binding to their receptors in those susceptible to AD. They also expect to stimulate results from these studies to start a revolution in the AD treatment. the natural form of amyloid formation consists of three phases; the first phase is related to the nucleus, which is a critical phase in amyloid formation, the next step is elongation and increase of fibril size. this phase is the most fast phase in which the fibril formation is formed rapidly and the level of fluorescence or absorption of indices such as thioflavin (THT) and congo-red (CR). the final phase of the equilibrium phase is equilibrium through which the level of fluorescence or absorption of specific amyloid plaques is usually not altered, and the system seems stable. however, researchers have shown that fibrils are also active in this case and the monomers are exchanged. furthermore, the lateral interaction among the fibrils is formed and as a result, mature and thick fibrils are produced.
Today, gold, silica, silver and iron nanoparticles are widely used in the pharmaceutical industry worldwide. the medical use of these nanoparticles is always focused on different structures and also the ratio of surface to volume ratio and penetration power to live tissues that are considered as the main properties of nanoparticles. magnetic nanoparticles, for example, trivalent iron oxide nanoparticles or gold. The reason we used magnetic nanoparticles was the ability to track NP, as it is known, paramagnetic iron nanoparticles have magnetic properties.
Diagnosis of targeted disease
Drug delivery systems based on nanotechnology have improved the eye in drug treatments due to the increased amounts of drugs in blood flow, decrease of toxicity and halflifetime medicine. these properties are obtained from targeted drug transport, in which the role of magnetic nanoparticles (MNP) as drug carriers is stronger due to their unique characteristics in other nanomaterials. Thanks to nanotechnology, who have facilitated a wide range of diagnostic and diagnostic applications in diseases such as cancer, heart disease such as cancer, heart disease and anxiety [6].
Data analysis
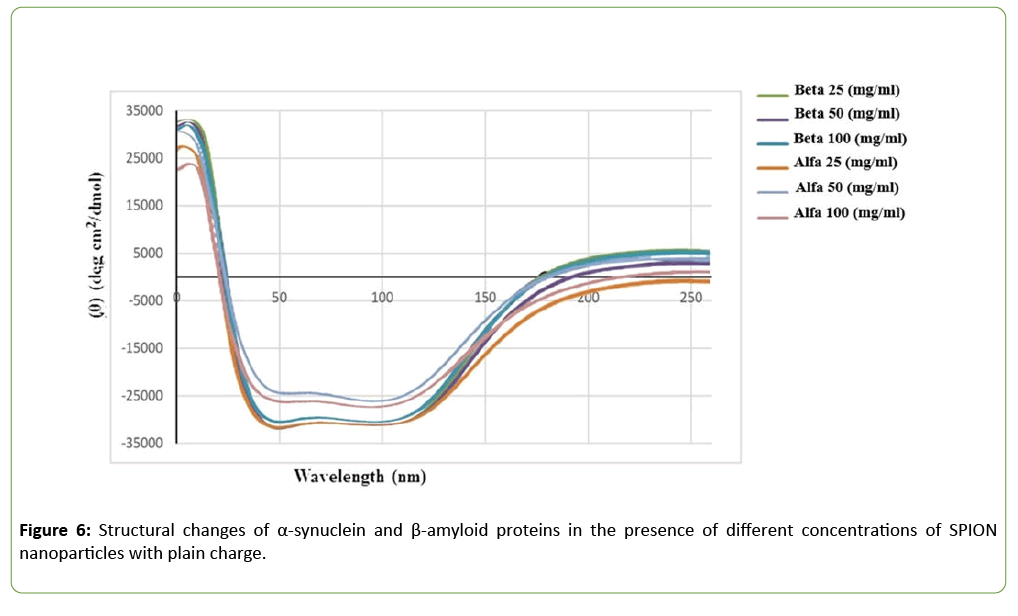
This article contains the data on the measurement of toxic effect of commercial superparamagnetic nanoparticles on SHSY5Y neuro cells using MTT method. As the main part, data of the influence of the commercial nanoparticles with various electrical charges and concentrations on transformation of the second structure of α-synuclein and β-amyloid proteins can be observed. Figures 1-4 include the graphs that illustrate the rate in which the neuro cells survive by the influence of the commercial SPIONs with different concentrations. Figures 5 and 6 provide diagrams of changes in the second structure of α-synuclein and β-amyloid proteins using circular dichroism test.
Materials and Methods
Experimental design: Investigation of cell survival in presence of SPIONs
MTT assay was used to evaluate the toxicity effect of nanoparticles and to assess the cell survival ability. In the MTT test, the SH-SY5Y neuronal cell line was purchased from the Cellular Bank of Pasteur Institute of Iran. The test was carried out by measuring the absorption of tetrazolium salt. To prepare the MTT storage solution, 5 mg of this compound was dissolved in 1 ml of phosphate saline buffer. The cells were cultured in 96 well houses with 10,000 cells per well and incubated at 37°C for 24 hours. After that the growth rate of the cells reached to about 70%, then the nanoparticles with positive, negative and neutral charges and different concentrations of 2.5, 5 and 10 mg/ml were added to the culture medium and the control well was considered with the culture medium without NPs effect. After 24, 48 and72 hours incubation in (5% CO2), 100 μl MTT was added and incubated for 2 hours, and then the culture medium was discarded. The reaction was stopped by adding DMSO. Because this solution dissolves the violet formazan sediment in the survived cells, hence the amount of absorption was measured by the ELIZA device (Assay Immunosorbent Linked Enzyme) at 570 nm [2]. In this study, the data were analyzed by 20 SPSS software through ANOVA test and the significance level was measured on p<0.05.
Structural changes investigation by utilizing Circular dichroism
In order to demonstrate the changes in the second structure of α-synuclein and β-amyloid proteins in the presence of SPIONs with different concentrations, the Aviv Model 202 Circular dichroism device was used which show changes in the content of α-helix protein in the presence of SPIONs. During this experiment, SPIONs were incubated at different concentrations (2.5, 5 and 10 μl) separately with 100 μl α- synuclein and β-amyloid proteins at 37°C and after an hour incubation the amount of structural changes (the second structure) of these proteins were investigated. The analyze was carried out at wavelengths of 190-300 nm, and the peaks obtained were investigated in regions 208 nm and 220 nm.
Results and Discussion
Now more than 20 types of related diseases are known, including Alzheimer's (AD), Parkinson (PD), Huntington (HD), and so on. in these diseases, the complexity of the protein leads to the formation of Amyloid structures that are insoluble and soluble in cell. In fact, the monomers are first bound together to produce Oligomers and are then developed through the accumulation of oligomers (insoluble) structures. The compounds of enriched uranium are at β-plates and have 10-20 nm sizes [2].
The mechanism of Amyloid formation depends on the nuclease stage and consists of a slow phase called lag phase, which are related to each other through it. This phase, which determines the extent of the process, is looking for the elongation phase where fibril is formed by adding quickly to the sub-units [4]. Amyloids are of two types : Those who are composed of non-folded proteins such as Amyloids and α- synuclein, and proteins such as albumin, β-2 and Teresty retin (TTR), each of which form a Amyloid structure depending on the type of amino acids and peripheral conditions [4].
Albumin is one of the most important physiological protein that contains approximately 70% of the protein concentration in the plasma and is responsible for many vital functions in the body. this protein includes amino acid and disulfide bonds and can form amyloid fibrils in specific conditions. Infact, amyloid formation mechanism is different in various proteins, but they are different from different factors depending on the type of amino acids, structure and other environmental factors. however, the selection of albumin for amyloid protein fibrillation studies is due to its availability, availability and fibrillation conditions. in addition, bovine albumin protein is very similar to human albumin. So, the results of studies on this protein can be extended to human albumin [7].
Recent studies have shown that the interaction of nanoparticles with different proteins causes severe structural changes, as such changes can lead to the destruction of protein function or the unpredictable effects on the process of inducing ventricular fibrillation. Fibrils formation or fragmentation of different proteins in the presence of nanoparticles varies widely depending on protein type and chemical properties of nanoparticles [8].
Alzheimer's has now caught many people in the world. It is developed due to widespread destruction caused by neural connections between neural cells. When neural cells are destroyed, there is no way to cure these diseases. However, if they are detected prior to the destruction of nerve cells, AD may impede AD. The accumulation and formation of platelets can be very serious break the synapse and sever interpersonal communication. Alzheimer's disease (AD) is characterized by the accumulation of two proteins, the tau protein and tau protein, which are assembled inside and outside the neuron. more accumulation of colonies known as amyloid fibrils are highly toxic and cause dysfunction of neurons in neurons. there is a high concentration of tau protein in the ad hoc neuron known as accumulation of tau protein. in normal case, only some of these parts are present in the cells and they dialysis very fast. however, if the proteome of the neural cells is impaired and their level is increased, the structure of the spherical protein is formed and therefore ad can be developed [9].
Scientists and researchers believe that if the accumulation of Aβ is prevented, the development of Alzheimer's might be restrained. Almost 27 types of diseases related to protein structure exist in humans, including HD, PD and type II diabetes. Naturally, proteins A are present in form of α -helix structure, which is converted to β-sheets due to the defect of the structure. This happens when the folding process occurs in these wrong protein compounds. The sheets formed by β - Amyloids are the structures caused by the accumulation of toxic forms or insoluble fibrils. Normally, the defect in the formation of β-Amyloid occurs due to the substitution of amino acid sequences instead of 35-28 amino acids instead of 35-28 amino acids. This shift of amino acid sequence leads to the formation of β-Amyloid plates, which can cause various factors, including genetics agents, metal ions, etc. [10].
The present study examined the effect of para oxide nanoparticles on ventricular fibrillation, β-Amyloid and albumin. First, in order to fully understand the properties of nanoparticles, two types of nanoparticles were synthesized in this technique, which was done in reverse sedimentary method. Review of properties and properties of the trading nanoparticles and properties purchased by the techniques of diffraction patterns Scanning electron microscopy (ED: electron, TEM, and analysis of the chemical composition and crystal structure were observed with x-ray diffraction (XRD). also, in order to study the magnetic properties of magnetite nanoparticles, vibrating sample magnetic and magnetometer sample was used to study the magnetic properties of magnetite nanoparticles. electrical load rate of SPION nanoparticles was measured by Zeta technique. All the features of the purchased commercial nanoparticles have also been announced in the Appendix.
The study of the peptide process of β-peptide, α-Synuclein, and albumin is used by the fluorescence measurement technique of salt binding to β-plates in the Thioflavin T (THT) method. in order to study cell survival and cell culture, using the MTT test and examining the changes of secondary structures using (CD) and dichroism were studied.
In this study, iron oxide nanoparticles were used to consider the general feature of FeO2 (protein group loads). Zeta analysis of the purchased commercial nanoparticles showed that the SPION nanoparticles with positive loads (NH2), negative (COOH) and neutral (Plain) have magnetic loads.
In the present study, the effect of SPION nanoparticles on atrial fibrillation of Β-Amyloid and α-Synuclein was investigated. The role of a number of physical -chemical parameters such as the size and load of coated, as well as the effect of the magnetic field on lag time before the onset of ventricular fibrillation were investigated. it was found that particle size had a significant effect on the process of fibrillation, possibly due to the change in protein binding or alteration in protein composition. Indeed, SPION have dual effects, as the observed effects depend on the ratio of the surface of the particles to the protein concentration, which has a proper interaction of the concentration, shape and composition of the protein on the formation of the core nucleus. dependence on protein concentration is important in formation of cores. These are the appropriate items at the beginning of the initial phase in the ventricular fibrillation. Recent studies have shown that the interaction of nanoparticles with different proteins causes severe structural changes, as such changes can lead to the destruction of protein function or the unpredictable effects on the process of inducing ventricular fibrillation. Fibril formation or fragmentation of different proteins in the presence of nanoparticles varies widely depending our findings indicate that applying magnetic field to antagonists has an effect on fibrillation of proteins due to its effect on nanoparticle size due to surface charge. It was found that in magnetic field and low concentration of nanoparticles (SPIONs dextran-NH2), the process of fibrillation of proteins was accelerated while in higher fibrillation inhibited fibrillation. Furthermore, the charge -induced load on the nanoparticle has an important role in the process of fibrillation and has accelerated magnetic charge, at low concentration, fibrillation compared to negative and negative charges.
Although studies are still in their early years, SPION are recognized as a prominent candidate for multi-functional and biomedical applications, and the influence of the magnetic field on the SPION is particularly important. as mentioned earlier, the magnetic field has been used for the treatment of cytokines and the effect of antagonists on the pathogenesis of β-amyloid fibrillation has been studied. While the lower concentrations reduce the amount of ventricular fibrillation, higher concentrations increase the amount of ventricular fibrillation and α-Synuclein. Due to the load cover, it is evident that non-charge bonds have the ability to increase ventricular fibrillation compared to negatively charged SPION, indicating that in addition to the presence of nanoparticles, which affects the concentration of the monomer (nucleation time), there is also the effect of ionic connections on protein formation. With the effect of load-loaded nanoparticles at the beginning of the fibrillation process of the proteins at the initial and important phase of the process, i.e., initial phase or lag phase, this process is much longer than the onset of the process.
furthermore, after that, phase unwrapping has been continued with shorter absorption wavelength than positive control and because of the effect of COOH and COOH and with the effect of the effect of COOH and NH2 with the amino acid units of each of the studied proteins has been investigated.
Commercial nanoparticles purchased from the Micro mod Company are known to be very effective on the surface charge of nanoparticles and surface functional groups in the process of forming Amyloid proteins.
Tendency of inducing ventricular fibrillation, α-Synuclein and human albumin is investigated under different conditions of solution. The accumulation kinetics, the focal changes in the protein themselves, and the structure of different mediators on the trajectory of ventricular fibrillation (fluorescence) and absorption of Dichroism and electron microscopy were studied. the fibrillation of the protein was measured during incubation at neutral pH and body temperature. The process of ventricular fibrillation was accompanied by increasing rising in the creation of β-plates and irregular compatibility in the α- helical production, shown with fluorescence tests of THT and Circular Dichroism. These changes also accompanied the presence of various structural brokers on the direction of ventricular fibrillation.
According to our experiments in the Circular Dichroism test, to evaluate the effect of different concentrations of SPION nanoparticles on the secondary structure of α-Synuclein, β- amyloid, and albumin proteins, 25, 50 and 100 μg/ml of SPIONs were first tested. The cells were incubated for 5 hrs at 37°C with each of the proteins α-Synuclein, β-amyloid and albumin.
The presence of two negative peaks at 47 and 120 nm indicates the classical structure of the protein, which has α- helix structures and β-plates. Statistical analysis of ANOVA results shows that the difference of rotational radiation of the combination of β-amyloid protein and nanoparticles with all concentrations of 50.25 and 100 ml/gμ is greater than 0.05, therefore this difference in radiation Proteins and their specific concentrations are meaningless. Therefore, it can be said that the effect of SPION nanoparticles on the secondary structure of β-amyloid and α-synuclein proteins is visible in the circular dichroism test, but these changes are not statistically significant at any of the positive, negative and neutral concentrations and types of (p>0.05).
We propose that the cause of fibril formation may be due to the role of intermediates such as oligomeric, resulting in the cluster pathway through clustering of these oligomeric species to produce protofibrils and then fibrils. The resulting fibrils are thick and twisted and vary depending on the solution. In acidic conditions, circular fibers are usually observed if the fibers are flexible enough and long enough. These fibrils can be formed by a parallel arrangement of β-strands that form the structure of the β-plates in the proteins studied as the most likely configuration. The very long incubation time results in more complex morphological changes in mature amyloid fibrils, which must consider environmental conditions such as pH, temperature and to be able to position themselves in close proximity to each other. Paramagnetic iron oxide nanoparticles have iron oxide nuclei that can be attached to a specific part by magnetic coating. They have properties such as paramagnetic superconductors, high saturation field, non-toxicity, biodegradability, and variable rings. The toxicity assessment in the experiments has also shown that zinc-bound nanoparticles are more toxic than copper and silver nanoparticles, while iron and titanium nanoparticles have the least toxicity.
Nanoparticles having a hydrophobic nature tend to attach to the hydrophobic segments (amino acids) of proteins and those that are hydrophilic in nature tend to attach to hydrophilic amino acids, obviously the difference in binding and affinity constants. Nanoparticles for binding to amyloid proteins will be effective in the formation of amyloid protein fibrils. Depending on the protein type and the physical and chemical properties of the nanoparticles, the proteins bound to the surface of the nanoparticles undergo structural changes that affect the performance of these proteins and nanoparticles.
So our interpretation is that there are three regions in the α- synuclein protein: It is highly conserved called the N-terminal region, a non-amyloid-mediated variable region (NAC) responsible for fibrillation, and an acidic region up to Intact Cterminal that acts as a chaperone. The α-quinoline protein is neutralized at neutral pH but changes in structure toward acidification (pH) or metal ions and even oxidative stress. The prevalence of Alzheimer's at age 60 is about 5-10% higher, but dramatically increases at about 40-50% above age 85. Β- amyloid is an amphipathic polypeptide with a molecular weight of 4 kDa containing 1-42 amino acids and spontaneous folding of β-amyloid which causes fibrillation plays an important role in the onset and progression of Alzheimer's disease. The amyloid β-monomeric form is relatively soluble, with the remaining 16 subunits of the amyloid β-structure being highly soluble, however, the rest of the sequence contains two fragments of hydrophobic residues (16-21) and (30-42), which are believed to be Which play an important role in oligomerization and are separated by a region containing two acids (Glu 22 and Asp 23) and a base subunit (Lys 28). It has been suggested that hydrophobic nuclei play a key role in β-amyloid accumulation at the time of fibril formation. The results show that the application of magnetic field to SPIONs affects Aβ fibrillation because of its effect on the size of the nanoparticles due to surface charge. It was found that in the magnetic field and high concentration of nanoparticles (SPIONs dextran-NH2), the process of Aβ fibrillation accelerates while inhibiting fibrillation at lower concentrations. In addition, the charge on the nanoparticles plays an important role in the fibrillation process, and positive/magnetic SPIONs, at lower concentrations, accelerate fibrillation compared to neutral or negative SPIONs. Although studies are still in their infancy, SPIONs have been recognized as prominent candidates for multipurpose and biomedical applications, and the impact of the magnetic field on SPIONs is of particular importance. As mentioned earlier, the magnetic field has been used for the therapeutic use of SPIONs and the effect of SPIONs on the β- amyloid fibrillation process has been studied. While lower concentrations of SPIONs inhibit fibrillation, the concentrations Higher, increases the rate of Aβ fibrillation. With respect to the coating charge, it is clear that the positive bonds are capable of increasing fibrillation at low concentrations compared to non-charged or negative SPIONs, indicating that in addition to the presence of nanoparticles, it affects the monomer protein concentration in solution (e.g. Here at nucleation time There is also the effect of junctions on protein formation. As can be seen in the comparative diagram of the fibrillation processes of both proteins, the difference in fluorescence absorption levels between the two β-amyloid and α-Synuclein proteins was evident, with the difference of each type of charge at all three concentrations being compared with the statistical analysis. At 95% level it was meaningless. The reason for this qualitative difference in the greater inhibition of the nanoparticles in the β-amyloid protein can be attributed to the point of effect and the region responsible for fibril formation and assembly, namely the ablation region of 16 to 20 β-amyloid peptides, which is more readily available than the α-peptide peptide and even the peptide size. It allows the spay nanoparticles to exert their inhibitory effect on the β- amyloid peptide more than the α-Synuclein peptide. However, the main purpose of this paper is to investigate the effect of magnetic field on PEG coated SPIONs with different loads on the amyloid fibrillation process. SPION measurement properties (DLS) were performed using the Malvern Zetasizer Nano ZS90 tool.
It can be said that the identification of amino acids 64-64 from α-Synuclein as a region of its own entanglement. A region of α-Synuclein that may be involved in neurodegenerative disease is in a region called the non-amyloid β-component (NAC) that is isolated from patients with Alzheimer's disease that correspond to regions 61 to 95 of the α- amino acid. has it. Based on our results on the α-nuclease peptide, we conclude that the N-terminal region of the NAC, which is part of the 64 to 86 core junction, is responsible for its selfassembly and aggregation, and that the n-terminal region of 68 to 72 is the α-nuclease peptide hydrophobic region. Also, α- synuclein (66-74) has an important role in its accumulation and toxicity. Taken together, all of these results indicate that α- synuclein self-accumulation is mainly driven by hydrophobichydrophilic interactions. In the present study, the effect of SPION nanoparticles on Aβ fibrillation was investigated. The role of a number of physico-chemical parameters such as the size and charge of dextran coated SPIONs as well as the effect of magnetic field on lag time prior to the initiation of Aβ fibrillation was investigated. The particle size was found to have a significant effect on the Aβ fibrillation process, possibly due to changes in protein binding to the site or changes in protein composition. In fact, SPIONs have dual effects, in that the effects observed depend on the ratio of the surface area to the protein concentration, which has a favorable interaction with the concentration, shape and composition of the protein on core formation. Dependence on protein concentration is involved in the formation of nuclei. These are suitable cases for starting the initial phase in fibrillation processes. Another issue is the non-toxic characteristic of these nanoparticles. As reported, the use of NP in various studies has caused cytotoxicity in living cells. As a result, the use of tests and toxicity assessment techniques for drug and medical use of nanoparticles is recommended. Iron, gold, and silica nanoparticles have minimal levels of toxicity to living cells. The trivalent iron oxide nanoparticles used in our research are FDA approved by the Food and Drug Administration. In the present study on different nanoparticles (considering that the nanoparticles were loaded. The findings showed that negative nanoparticles were effective on the structural inhibition of α- fibers. In the present study, the minimum concentration of negatively charged nanoparticles encoded by COOH ions was 2.5 μl. The diagram obtained in the lag phase was considerably longer, in other words, that phase was delayed or inhibited, and eventually the nucleation phase became shorter. What is the consequence of shortening or decreasing the nucleation phase in the fibrillation process? This means that the number of β-plates formed by the process of mature fibrils forming our proteins is reduced, which means that less amount of THT is attached to positive controls and other loads. Therefore, the fluorescence absorption is less than in other graphs. The diameter of the nanoparticles used in our study was 20 nm, indicating a good absorption capacity for drug use. And finally, based on the results of investigating the mechanism of amyloid fibril formation in the two Aβ and α-synuclein proteins, it was found that the nanoparticles coated with negative concentration encoded with -COOH ions could decrease significantly from 17000 to 12000 after 320 minutes after the lag phase shows lag phase and decreased level of THT binding to β-plates. In the study of nanoparticles and their inhibitory effect on A-fibril formation, their surface-to-volume ratio and their influence on the interaction of fibril formation have been reported as the cause of the inhibitory effects. Drug use through a binding factor or carrier is recommended in various scientific articles. The reason for this is due to the good coverage of the nanoparticles and interaction with different materials and, of course, more and better bonding with these materials. Trivalent iron oxide nanoparticles are spherical, so they can have the best surface contact with their environment compared to non-spherical nano particles [11,12].
Conclusion
In this study, after studying the toxicity characteristics of nanoparticles and the toxicity level of nanoparticles due to different concentrations and loads, it can be concluded that the best result of application and selection of trivalent iron oxide paramagnetic nanoparticles is that it can be used as a Carrier drug that, with FDA approval, confirmed this certainty for the nanoparticles purchased from the present study. Since the amyloid proteins resulting from the fibrillation process are similar to the oligomers in terms of the structure of the β- plates, the amount of amyloid proteins that make up the fibrils can be identified using THT analysis. The marker was used to diagnose diseases and also to predict and detect the amyloid proteins of each patient to predict the fate and physiological response of patients to nanoparticles. Examine different patients. Since nanoparticles injection site has an important effect on the therapeutic efficacy and viability of nanoparticles in the body, it should be injected into different parts of the patient's body and examined its biological effects on different patients' body.
In order to evaluate the effects of different nanoparticles on patients, it is advisable to do all the in-housework done during the project. The formation of specific amyloid protein phenomena for other nanoparticles can also be investigated. The effect of changes in physical and chemical properties of nanoparticles on changes in the formation of specific amyloid aggregates in different amyloid diseases.
Acknowledgement
This research received financial support from the grant Number 91059044, by the office of presidency, vice presidency for science and technology, Iran National Science Foundation.
Conflict of Interest
There is no conflict of interest.
References
- Zarranz JJ, Alegre J, Gómez‐Esteban JC, Lezcano E, Ros R, et al. (2004) The new mutation, E46K, of α‐synuclein causes parkinson and Lewy body dementia. Ann Neurol 55: 164-173.
- Fink AL (1998) Protein aggregation: Folding aggregates, inclusion bodies and amyloid. Fold Des 3: R9-23.
- Kumar S, Udgaonkar JB (2010) Mechanisms of amyloid fibril formation by proteins. Curr Sci 98: 639-56.
- Anderson JP, Walker DE, Goldstein JM, De Laat R, Banducci K, et al. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281: 29739-29752.
- Mahmoudi M, Milani AS, Stroeve P (2010) Synthesis, surface architecture and biological response of superparamagnetic iron oxide nanoparticles for application in drug delivery: A review. Int J Biomed Nanosci Nanotechnol 1: 164-201.
- Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, et al. (2010) Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett 10: 2543-2548.
- Ferreira S, Duarte AP, Ribeiro MH, Queiroz JA, Domingues FC (2009) Response surface optimization of enzymatic hydrolysis of Cistus ladanifer and Cytisus striatus for bioethanol production. Biochem Eng J 45: 192-200.
- Mambule C, Ando Y, Anan I, Holmgren G, Sandgren O, et al. (2000) Enhancement of AA-amyloid formation in mice by transthyretin amyloid fragments and polyethylene glycol. Biochimica et Biophysica Acta 1474: 331-336.
- Miller EC, Teravskis PJ, Dummer BW, Zhao X, Huganir RL, et al. (2014) Tau phosphorylation and tau mislocalization mediate soluble Aβ oligomer‐induced AMPA glutamate receptor signaling deficits. Eur J Neurosci 39: 1214-1224.
- Anderson JP, Walker DE, Goldstein JM, De Laat R, Banducci K, et al. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281: 29739-29752.
- Skaat H, Shafir G, Margel S (2011) Acceleration and inhibition of amyloid-β fibril formation by peptide-conjugated fluorescent-maghemite nanoparticles. J Nanopart Res 13: 3521-3534.
- Lee DY, Khatun Z, Lee JH, Lee YK, In I (2011) Blood compatible graphene/heparin conjugate through noncovalent chemistry. Biomacromolecules 12: 336-341.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences