Tuning the Magneto Structural Properties of Nickel Ferrite Nanoparticles for Different Technological Applications
Saif Ullah1*, Muhammad Ibrar2 and Ghafar Ali3
1Department of Physics, Government College, Peshawar, Pakistan
2Department of Physics, Islamia College, Peshawar, Pakistan
3Department of Nuclear Science & Technology, PINSTECH, Islamabad, Pakistan
- *Corresponding Author:
- Saif Ullah
Department of Physics, Government College, Peshawar, Pakistan
Tel: 923149472027
E-mail: saifphysics3@gmail. com
Received Date: December 09, 2021; Accepted Date: December 23, 2021; Published Date: December 30, 2021
Citation: Ullah S, Ibrar M, Ali G (2021) Tuning the Magne to Structural Properties of Nickel Ferrite Nanoparticles for Different Technological Applications. Nano Res Appl Vol.7 No.12
Abstract
Magnetic Nickel Ferrite Nanoparticles (MNFNPs), a kind of soft materials possess amazing and attractive properties and have vital technological applications such as a contract agent for magnetic resonance imaging, as an anode in the lithium-ion battery, as a catalyst, sensors and magnetic memory devices and so on. The present study concentrates on the synthesis of Magnetic Nickel Ferrite Nanoparticles (MNFNPs) by the co-precipitation method at different ratios. The elemental composition, structure, and morphology of these nanoparticles were characterized using Energy-Dispersive X-ray Spectroscopy (EDS), X-Ray Diffraction (XRD), and scanning electron microscope (SEM) while magnetic properties were determined using a Vibrating Sagnetic Spectrometer (VSM). The resulting nickel ferrite NPs exhibit very interesting properties. The size of NPs was found to be in the range of 11.7 nm to 260 nm depending upon annealing temperature. It has been observed that size of NPs increasing linearly with temperatures for 700°C and 900°C only. These particles are super paramagnetic at room temperature. The coercivity of these nanoparticles was found to be 0.03 kOe, 0.013 kOe, and 0.003 kOe while saturation magnetization is reported as 36.53 emu/g, 33.29emu/g and 37.23 emu/g respectively. The low saturation magnetization is attributed to the inert/dead layer or disordered surface spin of these MNFNPs.
Introduction
Magnetic and structural properties of ferrite Nanoparticles (NPs) have exploited the scientific community during the last few years. It is well established fact that these Nanoparticles (NPs) have novel properties at the nanometer scale. Nanostructured materials can be fabricated from this small group of atoms. Nanoparticles are in effect a bridge between molecular or atomic structures and bulk materials. The size and shape-dependent properties are observed at nano scale dimensions which separate them from bulk materials having the same physical properties regardless of shape and size. The large surface area of these NPs is attributed to unexpected and interesting properties. These findings create a new and exciting possibility to alter the different properties of NPs by controlling the shape and size [1].
Magnetic Ferrite Nanoparticles (MFNPs) show special magnetic, structural, chemical, electrical, and mechanical properties and have a variety of favorable innovative applications in color imaging, hyperthermia, biomedical applications, optical devices, high-frequency devices, Ferro fluids, high density magnetic memory devices, and magneto caloric devices [2,3]. Accordingly, ferrite (Fe2O4) can be synthesized with different metallic cations (A: Ni, Cr, Co, Zn) and different synthesis methods. These compounds show a spinel structure. It consists of a cubic compact package of oxanions where octahedral and tetrahedral sites are occupied by different cations. The general formula for these compounds is AB2O4 where A and B are metallic cations with different oxidation states. For spinel structure, the general formula obtained is [A1-i2+B3+i] C [Ai2+B1-i2+ B3+] DO4 where C and D are suffixes for the tetrahedral and octahedral sites. The subscript i is used for inversion grade or rate. Hence, the spinel structure is termed normal for i=0, inverse for i=1, and mixed for 0<i<1. The evidence shows that the synthesis method or size of particles influences the type of spinel. Particularly, in the case of the ferrite spinel structure, the inversion grade depends on metallic cation A [4-10].
Nickel ferrite is soft ferromagnetic material having high electrical resistivity, low coercivity and low value of magnetization saturation. This results in making it a suitable material for the magneto-optical and magn etic applications. Nanostructured nickel ferrites can be used in telecommunication and electronic applications because it is the best core material having nearly zero hysteresis loss [11]. Nickel ferrites NPs possess high conductivity and high permeability at high frequency [12]. Nickel ferrites are well known for their catalytic, electrical, electronic, gas and humidity sensing properties [13,14]. The properties of these nickel ferrite NPs strongly depend upon crystallite size. It can be used as an anode in lithium-ion battery, contrast agents for magnetic resonance imaging and acts as a catalyst [15,16].
The structural and magnetic properties of nickel ferrite nanoparticles depend upon annealing temperature. It means that there is correlation between the magnetic and structural properties which are termed as Magneto structural properties. The nickel ferrite nanoparticles usually possess inverse or cubic spinal structure. The Ni2+ cations occupy the octahedral sites while Fe3+ ions are equally shared among the C and D sites. The final properties (magnetization saturation, coercivity, and remenant magnetization etc.) of material are determined on the basis of the composition of nickel ferrite nanoparticles and structure (inversion grade). Taking the different ratios of nickel ferrite nanoparticles during synthesis all the above properties can be tuned with different annealing temperatures for different applications.
Several methods have been taken into account for the design of magnetic NPs including the egg white method [17], hydrothermal process [18], spray pyrolysis [19], micro emulsion technique [20], sol-gel method [21], reverse micelle technique [22], evaporation condensation [23] and combustion method [24], temple-assisted electrochemical method. The major drawbacks of these methods are that precise control over the particle size is quite difficult [25]. The co-precipitation method is a wet-chemical method and has a certain edge over the other synthesis methods due to its simplicity, low cost, easily available and controllable synthesis parameters. This method has been utilized for the synthesis of magnetic NPs, as the precise control over the particle size and shape can help to the production of materials with attractive properties [26]. Due to control over the particle size of the sample, freedom from contamination, energy efficient, low temperature, does not involve use of organic solvents and homogenous mixing of components this method show superiority over the other synthesis methods [27]. That is why the co-precipitation method is adopted in this work for the synthesis of nickel ferrite NPs.
Materials and Methods
Following chemicals were used for the preparation of MNFNPs. All the chemicals were free from contaminations and were used without further purification.
• Nickel chloride (NiCl .6H O)
• Ferric chloride (FeCl .6H O)
• Sodium hydroxide (NaOH)
• Oleic acid (C H O)
• Ethanol (C H OH)
Preparation of Magnetic Nickel Ferrite Nanoparticles (MNFNPs)
The starting materials were Nickel chloride and Iron chloride while sodium hydroxide was used as a precipitating agent. To prepare the solution 1.1879 g of nickel chloride was dissolved in 25 ml of distilled water to make 25 ml of solution and 2.719 g of ferric chloride was dissolved in 25 ml of distilled water. Both the solution was properly mixed on a magnetic stirrer at 80°C for 40 minutes. Sodium hydroxide (precipitating agent) 3 g was mixed in 25 ml of distilled water and then it is added drop wise to the solution until a pH of greater than 12 is achieved. 3-4 drops of Oleic acid were added to the solution to avoid agglomeration. After stirring the brown precipitate was cooled to room temperature and washed several times with distilled water. The precipitate is then washed and centrifuged at 2000 rpm with ethanol for 15 minutes to remove extra impurities. The acquired precipitate is then placed in the oven and dried overnight at 80°C. The final product is then ground into powder and annealed at 500°C, 700°C, and 900°C for 10 hours to study particle size dependency, saturation magnetization and coercivity of these nanoparticles.
Results and Discussion
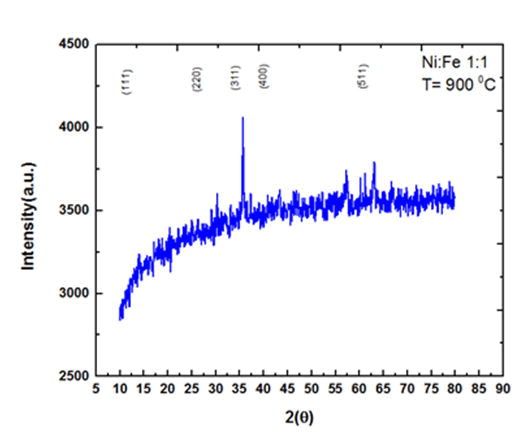
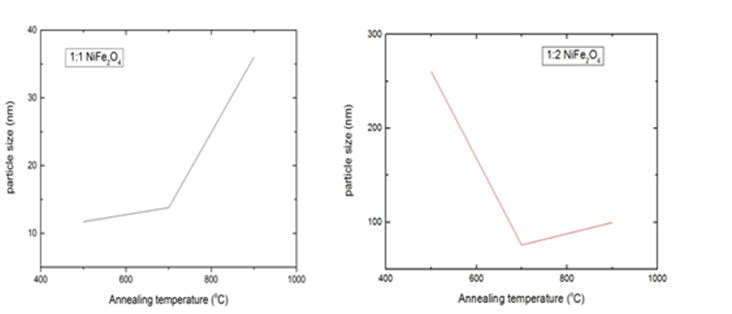
The crystal structure of prepared Magnetic Nickel Ferrite Nanoparticles (MNFNPs) in the range of 5°C to 90°C is shown in Fig.1. The peaks reported at 2θ correspond to the cubic spinal structure of MNFNPs. The diffraction peaks correspond to lattice planes (111), (220), (311), (400) and (511). XRD spectrum shows that the sample has good crystallinity and no impurity peaks. The average particle size was calculated by Debye Scherer equation (D=0.9λ/βcos θ), where D is crystallite size, β is line broadening at Full Width at Half Maximum (FWHM) of intense peak (311), λ is the wavelength of x rays and θ is Bragg's angle. The average crystallite size for 1:1 sample of MNFNPs was reported to be 11.7 nm, 11.7 nm, and 36.0 nm annealed at 500°C, 700°C, and 900°C however for 1:2 sample of MNFNPs the average crystallite size was found to be about 260 nm, 75.98 nm and 100 nm annealed at 500°C, 700°C, and 900°C.
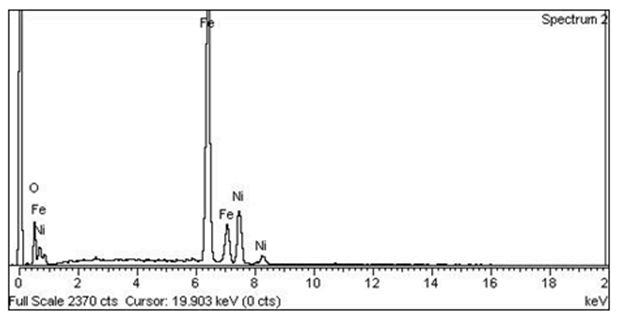
Energy-Dispersive X-ray Spectroscopy (EDS) is the technique used to carry out chemical characterization or to find out the elemental composition of the sample. The existence of nickel, iron, and oxygen in the structure of prepared Magnetic Nickel Ferrite Nanoparticles (MNFNPs) was confirmed by this technique. Fig.2 depicts the elemental composition of nickel, iron, and oxygen is 25.74%, 70.83%, and 3.43% respectively which are illustrated in table 1.
| Nickel ferrite nano particles (NiFe2O4) | Nickel | Iron | Oxygen | |||
|---|---|---|---|---|---|---|
| Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% | |
| Tann = 900°C | 25.74 | 22.82 | 70.83 | 66.03 | 3.43 | 11.15 |
Table 1: Elemental composition of nickel, iron and oxygen.
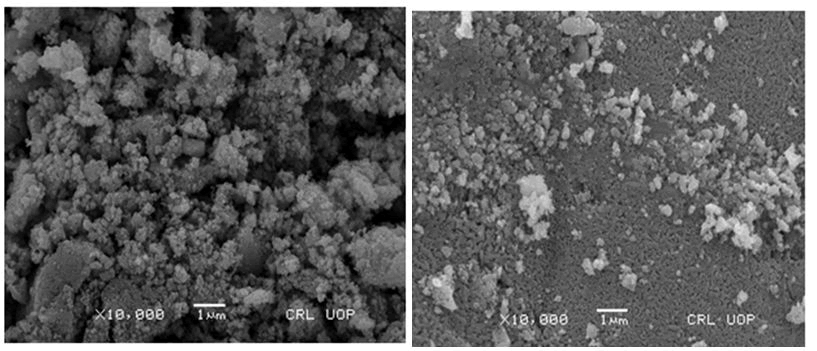
Fig. 3 and 4 depict the scanning electron micrograph of synthesized Magnetic Nickel Ferrite Nanoparticles (MNFNPs) for a 1:1 and 1:2 sample annealed at 900°C. SEM micrographs show that samples contain an aggregation of minute particles with elongated morphology, frequently distributed in the whole area and high dense agglomeration. Some separated particles are also indicated in the micrographs. This high dense agglomeration is due to the fact that NPs possess high surface energies. The average particle size of NPs for both the ratio was observed to be less than 100 nm however for 1:2 500°C sample the average particle size is 260 nm. The average particle size of 1:1 NiFe2O4 NPs annealed at 500°C, 700°C, and 900°C for 10 hours is about 11.7 nm, 13.8 nm, and 36.0 nm respectively while the average particle size of 1:2 NiFe2O4 NPs annealed at 500°C, 700°C and 900°C for 10 hours is about 260 nm, 75.98 nm, and 100 nm respectively. Fig.5 and 6 depict the plot of particle size versus annealing temperature. It also clearly presents that the size of NPs increases with an increase in annealing temperature for 1:1 and 1:2 samples annealed at 700°C and 900°C only. Also, the average size of NPs is larger for 1:2 samples as compared to 1:1 samples. Moreover, the linear dependency is not obeyed at 500°C for both 1:1 and 1:2 samples. Previously it has been observed that an increase in annealing time and annealing temperature produce coalescence of small grains that result in an increase in average particle size. Thus it is reported that particle size may be controlled by varying iron content in the sample, annealing timeor annealing temperature during the preparation of nanoparticles.
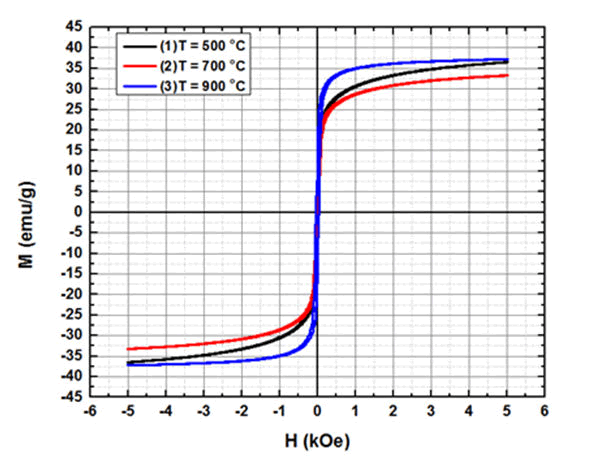
The magnetic measurements of the particles were performed using a Vibrating Sample Magnetometer (VSM) at a maximum applied field of 5 kOe. Fig.7 shows M (H) loops obtained from VSM for Magnetic Nickel Ferrite Nanoparticles (MNFNPs) at room temperature 300 K. The magnetic nature of NiFe2O4 NPs is very sensitive to particle size and crystallinity. The saturation magnetization (Ms), Remanent magnetization (Mr) and Coercivity (Hc) for 11.7 nm particles at an annealing temperature of 500°C as derived from the M (H) curve was 36.53 emu/g, 8.92 emu/g and 0.03 kOe. However, the Ms, Mr, and Hc values for 13.8 nm particles annealed at 700°C were 33.29 emu/g, 9.99 emu/g and 0.013 kOe while for 36.0 nm particles annealed at 900°C the Ms, Mr, and Hc values were 37.23 emu/g, 16.99 emu/g, and 0.003 kOe respectively. These magnetic parameters are listed in Table 2 given below. We have observed that the coercivity decreases with an increase in annealing temperature while saturation magnetization show the linear relationship with annealing temperature for 700°C and 900°C only. The values of Ms for NiFe2O4 NPs are significantly smaller than multi domain bulk particles (55 emu/g) Wijin et al. have observed Ms equal to 50 emu/g for bulk NiFe2O4 [28]. The negligible coercivity (Hc=0.03 kOe), (Hc=0.013 kOe), (Hc=0.003 kOe) and S shape of the M (H) loops is evident for the presence of magnetic particles possessing super paramagnetic nature [29]. The difference in magnetic parameters is attributed to the difference in structural morphology of magnetic nanoparticles.
| Temperature | Magnetic saturation(Ms) | Remenant magnetization(Mr) | Coercivity(Hc) |
|---|---|---|---|
| 500°C | 36.53 emu/g | 8.92 emu/g | 0.03 kOe |
| 700°C | 33.29 emu/g | 9.99 emu/g | 0.013 kOe |
| 900°C | 37.23 emu/g | 16.99 emu/g | 0.003 kOe |
Table 2: Magnetic parameters of NiFe2O4 nanoparticles samples (1:1).
Conclusion
In this work NiFe2O4 NPs of different ratios i. e. 1:1 and 1:2 were synthesized using co-precipitation method. XRD results reveal the preparation of pure NiFe2O4 NPs annealed at 500°C, 700°C and 900°C respectively. The sizes of NPs were computed by both XRD and SEM and were found to be in the range of 11.7 nm to 260 nm. These results were found to be in good agreement with each other. It is evident that the size of NPs increases with increase in annealing temperature at 700°C and 900°C only. SEM results reveal that most of the particles have spherical and elongated morphology with intense agglomeration. The room temperature VSM results show that NiFe2O4 NPs possess super paramagnetic nature with Ms values equal to 36.53 emu/g, 33.29 emu/g, and 37.23 emu/g annealed at 500°C, 700°C, and 900°C respectively. These values are much smaller than that of bulk values for NiFe2O4. The Hc values were found to be 0.03 kOe, 0.013 kOe and 0.003 kOe annealed at 500°C, 700°C, and 900°C respectively. It is surprising to note that such a particle sizes, small values of Ms and Hc for the above three annealing temperature and co-precipitation method have not yet been reported. By varying the annealing temperature, taking different ratio of iron content in chemicals, the particle size, shape, structure, and magnetic properties of Magnetic Nickel Ferrite Nanoparticles (MNFNPs) can be tuned for different technological applications.
References
- Rane AV, Kanny K, Abitha VK, Thomas S (2018) Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials (pp. 121-139). Woodhead Publishing
- Sharifi I, Shokrollahi H, Amiri S (2012) Ferrite-based magnetic nanofluids used in hyperthermia applications. J Magn Magn 324(6):903-915
- Maaz K, Mumtaz A, Hasanain SK, Bertino MF (2010) Temperature dependent coercivity and magnetization of nickel ferrite nanoparticles. J Magn Magn 322(15):2199-2202
- Larumbe S, Gomez PC, Pérez lJI, García PA, Alonso J, et al. (2012) Ni doped Fe3O4 magnetic nanoparticles. J Nanosci Nanotechnol 12(3):2652-2660.
- U Lüders, M Bibes, JF Bobo, M Cantoni, R Bertacco, J Fontcuberta (2005) Phys Rev B 71:134419
- J Jacob, MA Khadar (2010) J Appl Phys 107:114310
- MK Roy, HC Verma (2006) J Phys Condens Matter 18:7273
- L Wang, J Ren, Y Wang, X Liu, Y Wang (2010) J Alloy Compd 490,656
- DS Mathew, RS Juang (2007) Chem Eng J 129:51
- Larumbe S, Gomez PC, Pérez LJI, García PA, Alonso J, et al. (2012) Ni doped Fe3O4 magnetic nanoparticles. J Nanosci Nanotechnol 12(3): 2652-2660
- Jacob J, Khadar MA (2010) Investigation of mixed spinel structure of nanostructured nickel ferrite. J Appl Phys 107(11):114310
- Mirgorod YA, Borshch NA, Fedosyuk VM, Yurkov GY (2013) Magnetic properties of nickel ferrite nanoparticles prepared using flotation extraction. Inorg Mater 49(1):109-114
- Benrabaa R, Boukhlouf H, Löfberg A, Rubbens A, Vannier RN, et al. (2012) Nickel ferrite spinel as catalyst precursor in the dry reforming of methane: synthesis, characterization and catalytic properties. J Nat Gas Chem 21(5):595-604
- Jacob J, Khadar MA (2010) Investigation of mixed spinel structure of nanostructured nickel ferrite. Appl Phys 107(11):114310
- Zhao H, Zheng Z, Wong KW, Wang S, Huang B, et al. (2007) Fabrication and electrochemical performance of nickel ferrite nanoparticles as anode material in lithium ion batteries. Electrochem commun 9(10):2606-2610
- Ahmad T, Bae H, Iqbal Y, Rhee I, Hong S, et al. (2015) Chitosan-coated nickel-ferrite nanoparticles as contrast agents in magnetic resonance imaging J Magn Magn 381:151-157
- Maensiri S, Masingboon C, Boonchom B, Seraphin S (2007) A simple route to synthesize nickel ferrite (NiFe2O4) nanoparticles using egg white. Scriptamaterialia 56(9):797-800
- Nejati K, Zabihi R (2012) Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem Cent J 6(1):23
- Hong D, Yamada Y, Sheehan M, Shikano S, Kuo CH, et al. (2014) Mesoporous nickel ferrites with spinel structure prepared by an aerosol spray pyrolysis method for photocatalytic hydrogen evolution. ACS Sustain Chem Eng 2(11):2588-2594
- Misra RDK, Kale A, Srivastava RS, Senkov ON (2003) Synthesis of nanocrystalline nickel and zinc ferrites by microemulsion technique. Mater Sci Technol 19(6):826-830
- Chen DH, He XR (2001) Synthesis of nickel ferrite nanoparticles by sol-gel method. Mater Res Bull 36(7-8):1369-1377
- Kale A, Gubbala S, Misra RDK (2004) Magnetic behavior of nanocrystalline nickel ferrite synthesized by the reverse micelle technique. J Magn Magn 277(3):350-358
- Maaz K, Karim S, Mumtaz A, Hasanain SK, Liu J et al. (2009). Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route. J Magn Magn 321(12):1838-1842
- Mahmoud MH, Elshahawy AM, Makhlouf SA, Hamdeh HH (2013). Mössbauer and magnetization studies of nickel ferrite nanoparticles synthesized by the microwave-combustion method. J Magn Magn 343:21-26
- Maaz K, Karim S, Mumtaz A, Hasanain SK, Liu J, et al. (2009) Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route J Magn Magn 321(12):1838-1842
- Amighian J, Mozaffari M, Nasr B (2006) "Preparation of nano-sized manganese ferrite (MnFe2O4) via coprecipitation method." Phys Status Solidi Curr Top Solid State Phys vol.3 no.9:3188–3192
- Rane AV, Kanny K, Abitha VK, Thomas S (2018) Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. Elsevier Ltd.
- Smit J, Wijn HPJ (1959) Ferrites Philips Technical Laboratory
- Manova E, Tsoncheva T, Estournès C, Paneva D, Tenchev K, et al. (2006) Nanosized iron and iron–cobalt spinel oxides as catalysts for methanol decomposition. Applied Catalysis A: General 300(2):170-180
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences