Obtention by Coprecipitation and Magnetic Characterization of Fe3o4 Nanoparticles Coated with Surfactants
Priscila Chaves Panta and Carlos Pérez Bergmann
Priscila Chaves Panta*, Carlos Pérez Bergmann
Laboratory of Ceramic Materials, Federal University of Rio Grande do Sul, Av. Osvaldo Aranha 99/705C, Porto Alegre, RS, Brazil
- *Corresponding Author:
- Priscila Chaves Panta
Laboratory of Ceramic Materials, Federal University of Rio Grande do Sul, Av. Osvaldo Aranha 99/705C, Porto Alegre, RS, Brazil|
Tel: +55 51 33083637
Fax: +55-51-33083405
E-mail: pr.priscila@gmail.com
Received date: October 15, 2015, Accepted: November 30, 2015, Published date: December 04, 2015
Citation: Panta PC, Bergmann CP. Obtention by Coprecipitation and Magnetic Characterization of Fe0O4 Nanoparticles Coated with Surfactants. Nano Res Appl. 2015, 1:1.
Abstract
Nanostructured monodisperse particles, and magnetite (Fe3O4) were obtained by aqueous coprecipitation and surfactants coated with oleic acid and polyethylene glycol to control and ensure a uniform size distribution, in addition to reducing the effects of aggregation. A detailed investigation of morphological and microstructural characteristics was performed using XRD and SEM. The Raman spectroscopy showed peaks for magnetite (Fe3O4) and FTIR showed the interaction of Fe3O4 and its coatings, OA and PEG. The results show that Fe3O4 coated particles have an average crystallite size between 4.4-12.6 nm nanoparticles and less than the aggregate particles without coating. With the coatings, the morphology became more uniform the sizes of NPs. The magnetic properties of Fe3O4 coated particles were analyzed by AGFM. The hysteresis curves indicate a superparamagnetic behavior at room temperature, NPs PEG show a higher saturation magnetization while NPs OA. They also exhibit superparamagnetic nature at room temperature.
Keywords
Magnetite; Coprecipitation; Polyethylene glycol; Oleic acid; Magnetic properties
Introduction
In the last 20 years, the synthesis and characterization of nanomaterials has been a major focus of research because of the different physicochemical properties that the structures present in the nanometer range [1]. Among these nanomaterials, nanostructured Fe3O4 particles have attracted great attention because of their magnetic properties, such as superparamagnetism, high coercivity, low Curie temperature and high magnetic susceptibility, among other properties [2-3]. Nanoparticles (NPs) of Fe3O4 are promising for such applications as magnetic resonance systems, imaging for clinical diagnosis, recording materials, hyperthermia, drug delivery systems and catalysts [4-7].
Iron oxide magnetic nanoparticles have been the object of great interest in biomedical applications, since they can be susceptible Obtention by Coprecipitation and Magnetic Characterization of Fe3O4 Nanoparticles Coated with Surfactants to further functionalization with biologically active agents. These particles have the capacity to interact in different ways with various biological molecules [4] due to their superparamagnetic properties, high specific area and wide variety of surface functionalization [1-2]. Its surface can be easily modified by adding organic layers with polymers or inorganic oxides with metals or [4]. A wide variety of polymers such as hydroxy, carboxylate, styrene, vinyl alcohol or carboxy groups have been used as coatings [8] in the production of magnetic NPs. The coating/encapsulation of particles with polymers is the oldest and simplest method of preparation of magnetic particles [8].
Recent studies have shown different applications to oleic acid (OA) and polyethylene glycol (PEG) can be used as surfactants for coating iron oxide particles, serving as stabilizing agent. When coated with PEG [8] or OA [9], the nanostructured particles of Fe3O4 become biocompatible and acquire the ability to interact in various ways with various biological molecules [4]. Due to hydrophobicity of these particles, they become able to interact with the aqueous medium, but they are incompatible with organic liquids. Studies have been developed to enhance the solubility of the iron oxide NPs in apolar medium by OA preventing the agglomeration of these particles. PEG due to its hydrophilicity, non-toxicity, antigenicity, immunogenicity, can be connected to NPs because the ability for applications such as in drug delivery devices.
This study aimed to investigate the obtainment of Fe3O4 particles by the co-precipitation method and the effects of coating with OA [9] and PEG [8] to improve biocompatibility and the particles dispersion, and the study of the magnetic behavior. The conditions for obtaining the nanoparticles as a function of time and temperature synthesis and the coating (PEG and AO) were varied. The following measures were carried out X-ray diffraction (XRD), infrared spectroscopy (FTIR), Raman spectroscopy and electron microscopy (SEM) for the structural characterization of NPs produced. The magnetic properties were analyzed by magnetometry alternating gradient field (AGFM).
Synthesis and Characterization
Fe3O4 was synthesized by coprecipitation, according to the equation: Fe2+ + 2Fe3+ + 8OH- → Fe3O4 + 4H2O, keeping the molar ratio 1:2 for complete precipitation of Fe3O4 in a reducing environment, for magnetite does not become maghemite [10-12]. Salts, ferric chloride hexahydrate (FeCl3.6H2O, >99%) and ferrous chloride tetrahydrate (Fecl2.4H2O, >99%) supplied by Sigma-Aldrich® were employed as precursors. These salts were dissolved in deionized and deaerated water (40 ml) [13]. An inert gas (argon 99.99%, supplied by Linde) was passed through the aqueous solution of the salts under vigorous stirring of 400 rpm for a certain period (30, 60, 90 or 120 min) and under heating (60, 70, 80 and 90°C). When the established temperature was reached, 100 ml of tetramethylammonium hydroxide (TMAH, 25%) provided by VETEC® was dripped into the solution until reaching a pH of 11 and until it changed its color from brown to black, indicating the formation of Fe3O4 [2]. The Figure 1 shows experimental arrangement.
Before coating, the obtained particles were washed with deionized and deaerated water. For NPs coated with polyethylene glycol dripped with 15 ml of PEG 400, HO(CH2CH2O)n, with stirring, at 110°C for 1 h, after washing is made with distilled water. For NPs coated with oleic acid dripped with 20 mL of OA, C18H34O2, at a temperature of 100°C for 40 min and then make up the washing with isopropyl alcohol. The coated Fe3O4 particles were washed with ethanol/water to remove excess coating and unreacted monomers. In both coatings, it is the separation of the solution and the magnet with NPs and then, drying in an oven at 100°C for 6 h.
The characterization of the coated particles was performed by X-ray diffraction using a Philips-X'Pert MPD monochromator (CuKα, λ=0.15418 nm, 40 kV, 40 mA) with graphite. The morphology was observed by a scanning electron microscope (SEM, JEOL-JSM-6060), with maximum operating voltage of 30 kV and nominal resolution of 3.5 nm. The graphitization degree was measured in a spectrometer (FTIR, Perkin-Elmer Spectrum 100) in the range between 4000-500 cm-1. Raman spectra were recorded at room temperature by a Raman spectrometer (Renishaw Raman-inVia) with a linear laser excitation of 632.8 nm (He-Ne). An alternating gradient force magnetometer (AGFM) was used to measure M-H curves under a magnetic field range 0~1 T.
Results and Discussion
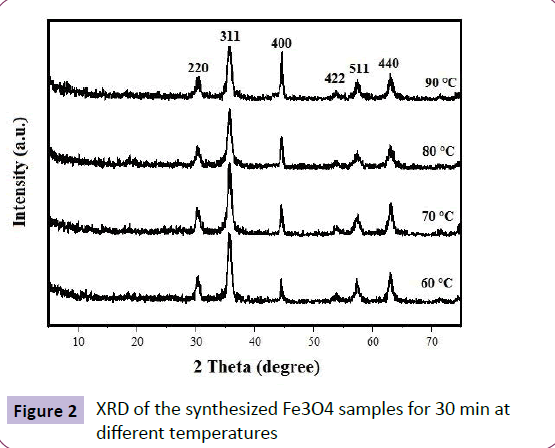
Using the coprecipitation method, a mixture of Fecl2 and FeCl3 powder was prepared at a molar ratio of 1:2 at different temperatures. Figure 2 shows two diffraction peaks of 2θ values at 35.87° and 63.02° associated with the Fe3O4 phase and Bragg reflections, according to the crystallographic planes (JCPDS 19- 0629), which corresponds to an inverse spinel structure [13-14].
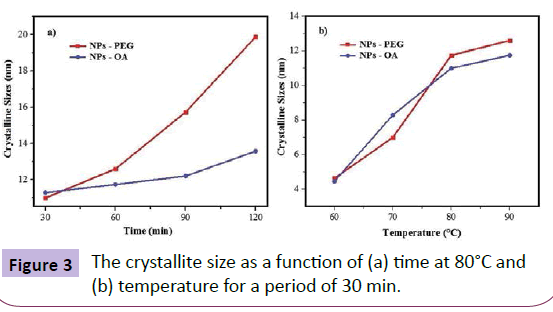
Figure 3 shows the mean crystallite size of the Fe3O4 nanoparticles, calculated using the Scherrer equation
D=K.λ/b.cosθ (1)
The equation uses the reference peak width at an angle of diffraction θ, where λ is the wavelength of X-ray (1.5418 Å), b is the peak width at half height of a selected reflection plane XRD and K is a factor due to the shape of the material, of about 0.94 to magnetite and maghemite, was estimated from these average crystallite size for the samples. According to Figure 2, an increase of the synthesis temperature by co-precipitation is increased crystallinity, where for each sample different temperatures the number of counts was the same. The XRD curve according to the half-height width peak of refraction plane (311) [1]. As can be seen in Figure 3a, the crystallite size increases significantly when NPs are synthesized for more than 60 min, and the increase is even greater for the PEG coated samples. In Figure 3b, the change in the crystallite size decreases significantly for NPs synthesized between 80 and 90°C.
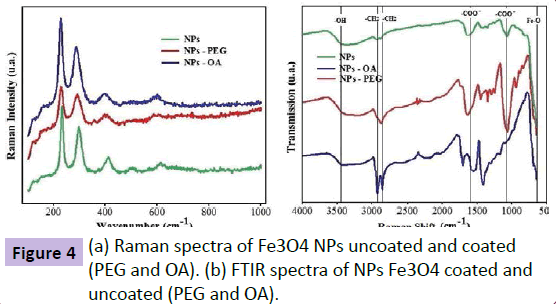
In Figure 4a, the Raman spectra of the Fe3O4 NPs show the characteristic bands for magnetite, according to Slavov L et al. The vibration peaks of the coatings (PEG and OA) are not observed. The FTIR spectrum (Figure 4b) obtained is characteristic of magnetite (Fe3O4), according to Yue-Jian C et al. [15] and Zhang et al. [16]. The magnetite characteristic peak at about 585 cm- 1, which is the band corresponding to the vibration of the Fe-O, confirming the presence of an iron oxide. The NPs of iron oxide coated with OA have vibration bands at 2850 cm-1 and 2920 cm-1 increased. As Bloemen M. et al. [17], adsorption bands of oleic acid corresponds to peaks in 2920 cm-1, 2850 cm-1 and 1435 cm-1 corresponding to CH2 vibration, the peak 1700 cm-1 relating to vibration C=O and the peak 1600 cm-1 relating to vibration -COOwas also oxidized. These results indicate that possibly the oleic acid was chemically adsorbed on the nanoparticle Fe3O4 [18]. The NPs of iron oxide coated with PEG exhibit vibration bands at 2860 cm-1 and 1435 cm-1 which represents -CH2 reinforced vibration. The peaks 1060 cm-1 corresponding to CH2-O-CH2 and 1195 cm-1 which corresponds to O-CH3 are characteristics of PEG.
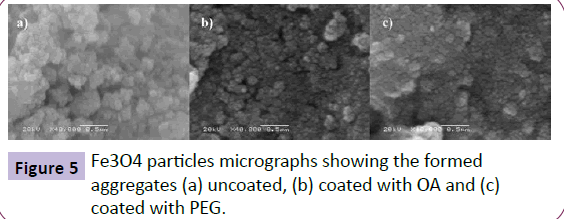
The images obtained by SEM in Figure 5 indicate the formation of packets of aggregates because of the higher surface energy of the nanoparticles. The uncoated NPs are even more aggregated than the coated NPs. The crystallite size was calculated by XRD, indicating that the obtained NPs have a polycrystalline nature, consisting of a large number of smaller sub-particles of dozens of nanometers.
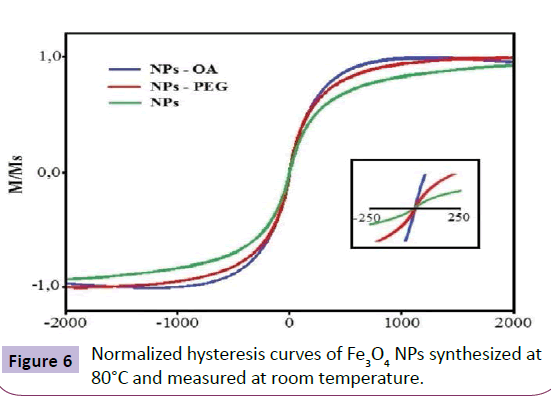
Magnetization measurements were performed to establish the magnetic field at room temperature. The hysteresis loops of coated (OA and PEG) and uncoated samples are shown in Figure 6. For a given magnetic field variation one can observe that the uncoated particles are less magnetized than the coated particles. The surface or magnetic properties of Fe3O4 are not always limited by the molecules of the coating. However, for the NPs-OA no saturation magnetization occurs. The particles have a superparamagnetic character at room temperature because of the absence of hysteresis and the average crystallite size. This means that in the absence of a magnetic field exhibit no magnetic remanence, leading to an overall magnetization unchanged [8,9].
Conclusion
Magnetic nanoparticles of iron oxide are synthesized into coated with polyethylene glycol and oleic acid, from the simultaneous precipitation of ions Fe+2 and Fe+3 in alkaline solution (NH4OH). The iron oxide magnetite has the characteristics according to Raman spectroscopy and XRD. The presence of the coating was confirmed by FTIR. The crystallite particle size ranged between 4.4 and 12.6 nm with larger aggregates tend to form when the particles are uncoated. SEM shows the tendency to form agglomerates due to the magnetic character. And the AGFM, there is a greater magnetization of the nanoparticles coated with PEG than OA, making more uniform morphology and size in both coatings, and a super paramagnetic behavior at room temperature.
Acknowledgement
The authors would like to acknowledge CNPq for financial support.
References
- Wu W, He Q, Jiang C (2008) Nanoscale Res Lett 3:397-415.
- Sun J, Zhou S, Hou P, Yang Y, Weng J, et al. (2007) J Biomed Mater Res 80:333-341.
- Chen ZP, Zhang Y, Zhang S, Xia JG, Liu JW, et al. (2008) Colloids Surf A 316:210-216.
- Laurent S, Forge D, Port M, Roch A, Robic C, et al. (2008) Chem Rev 108:2064-2110.
- Noginov MM, Noginova N, Amponsah O, Bah R,Rakhimov R, et al. (2007) J Magn Magn Mater 320:2228-2232.
- Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V (2005) Mol Pharm 2:194-205.
- Dalt SD, Panta PC, Toniolo JC (2011) Nanomagnetic Materials. Nanostructured Materials for Engineering Applications. Springer-Verlag 1:23-39.
- Jiang W, Yang HC, Yang SY, Horng HE, Hung JC, et al. (2004) J Magn Magn Mater 283:210-214.
- Gyergyek, S, Makovec D, Drofenik M (2011) J Colloid Interface Sci 354:498.
- Kim DK, Mikhaylova M, Zhang Y, Muhammed M (2003) Chem Mater 15:1617-1627.
- Sun YK, Ma M, Zhan Y, Gu N (2004) Colloids Surf A: Physicochem Eng Aspects 245:15-19.
- Zhou C, Wang H, Lou S, Li S, Du Z, et al. (2008) J Colloid Interface Sci 327:466-471.
- Marangoni VS, Martins MVA, Souza JA, Oliveira Jr ON, Zucolotto V, et al. (2012) J Nanopart Res 14:769-779.
- Ben Fredj H, Helali S, Esseghaier C, Vonna L, Vidal L, et al. (2008) Talanta 75:740-747.
- Yue-Jian C, Juan T, Fei X, Jia-Bi Z, Ning G, et al. (2010) Drug Dev Ind Pharm 36:1235-1244.
- Zhang L, He R, Gu HC (2006) Appl Surf Sci 253:2611-2617.
- Slavov L, Abrashev MV, Merodiiska T, Gelev C, Vandenberghe RE, et al.(2010) J Magn Magn Mater 332:1904-1911.
- Bloemen M, Brullot W, Luong TT, Geukens N, Gils A, et al. (2012) Nanopart Res 14:1100.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences