Importance of Nanoparticles in the Development of Durable Multi-functional and Smart Organic Coatings
Shalini Dolai and AS Khanna
Shalini Dolai* and AS Khanna
Department of Metallurgical Engineering and Materials Science IIT Bombay, Powai, India
- *Corresponding Author:
- Shalini Dolai
Department of Metallurgical Engineering and Materials Science IIT Bombay, Powai, India
Tel: 912225764618
E-mail: 133110055@iitb.ac.in
Received date: December 22, 2015; Accepted date: January 05, 2016; Published date: January 10, 2016
Citation: Dolai S, Khanna AS. Importance of Nanoparticles in the Development of Durable Multi-functional and Smart Organic Coatings. Nano Res Appl. 2016, 2:1.
Abstract
Importance of nano particles in formulating durable, protective and multifunctional coatings has been discussed in this review. Use of nano materials in organic coatings has brought a break-through in the coatings industry. Development of advanced coatings like multi-functional and smart coatings has been escalated with the introduction of nano technology into coatings. Different types of nano particles contribute different functions and these properties have been reviewed. Mechanisms involved in inducing each property and recent advancements in each area have been discussed briefly. Recent literature published on the use of nano particles in organic coatings has been focused.
Keywords
Nanoparticles; Organic coatings; Titania (TiO2); Silane
Introduction
Nano particles are engineered structures at nano scale whose size ranges between 1 nm to 100 nm. These are used in coatings as filler materials, opacifiers and semiconductors. With relevance to the coating industries, nano particles have shown significant potential for much stronger, tougher and durable coatings. They offer unique contributions when dispersed in coatings. For example, they can be used for formulating functional smart coatings like Super hydrophobicity, anti-icing, anti-fogging, antireflective, anti-fouling, self-healing, anti-bacterial, anti-corrosion and corrosion detecting, anti-graffiti etc., [1]. These unique properties are the outcome of outstanding aspect ratio, excellent surface interaction and high modulus. Numerous papers have been published on the enhancement of performance properties of polymer coatings by the addition of nano-materials [1-4]. Due to the high surface area of the particles, even a small amount of addition can bring a big change in the performance of the coating. One can create magic using nano technology in the field of coatings [2]. This needs an understanding of material selection, size effect and importance of morphology of the nano particles. Different types of nano particles can be incorporated into the polymeric resins depending on the requirement and service environment. These nano particles enhance the properties by various mechanisms. They can be used for increasing resistance to shrinkage during curing, delaminating and blister formation and photo degradation. Some nano-particles like zinc oxide (ZnO), titania (TiO2) have large band gap energy and high refractive index act as UV blockers or absorbers when used as fillers [3]. One of the major problems faced in the use of nano particles in coatings is the dispersion. Because of the high surface energy, they tend to agglomerate. This problem can be overcome by using dispersing agents and methods like ultra-sonication or functionalization with silanes and flouro compounds. Compatibility of nano particles and the polymer matrix is also a very important factor. Functionalization of surface of the nano particles with silanes is the most common method followed to achieve compatibility, improve bonding and decrease agglomeration.
Among all the pigments, nano inorganic materials are considered to be most advanced to improve mechanical and performance properties. Nano TiO2, ZnO, (silica) SiO2, (alumina) Al2O3, carbon black etc., [1-3] are commonly used for serving different purposes. Each kind can perform more than one function [1]. For example, nano ZnO can be used as UV blocker but it can also improve the scratch and abrasion resistance. Hence, classification of nano particles based on function is difficult. Hence, each property is discussed separately and nano particles that can be used to achieve the particular property are discussed in this review.
Effect of nanoparticles on properties of organic coatings
Effect on weathering properties
Aromatic compounds like epoxy coatings are vulnerable to UV degradation. Aromatic ring in the epoxy coating opens up, forms quinone structure, and has inferior properties. The formation of quinone can be determined by yellowing and color change indices. Higher the index value more is the degradation. Yellowing index represents the tendency of a white epoxy coating towards yellowing. Yellowing index is derived from tristimulus values of CIE primaries and it is measured by equation (1) [5].
YI=100(1.28XCIE-1.06ZCIE)/YCIE (1)
Color change index is the overall change in the color of the coating. If ΔL, Δa , Δb represent the differences in whiteness, blue or yellow and green or red respectively of control sample and UV exposed, then color change index is given by equation (2) [5].
dE=[Δa2+Δb2+ΔL2]1/2 (2)
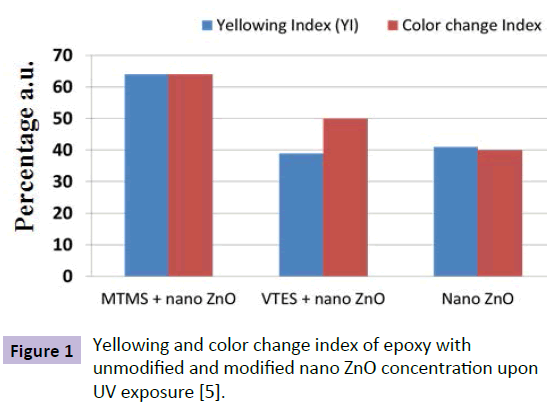
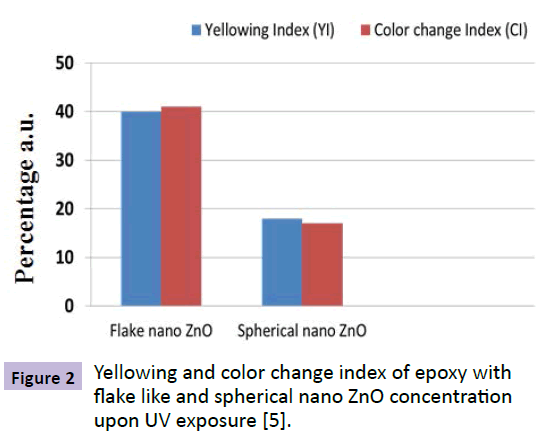
Nano TiO2 (rutile) and ZnO are commonly used UV blockers as the band gap energy of these materials is very high i.e., 3.2 eV to 3.4 eV [1,2]. Hence, they can absorb the UV radiation. Size, shape, crystal structure and concentration of the particle and its dispersion in the matrix play a major role. Narayani Rajagopalan et al. [4] investigated the effect of nano particles on epoxy. Epoxy, modified with silane and nano ZnO, showed 70% increase in the resistance to yellowing whereas epoxy with nano ZnO showed 40% increase under Xenon exposure. This shows the importance of compatibility between the coating and nano particles. Figure 1 shows the percentage increase in resistance to yellowing and color change of bare and modified epoxy coatings noted after 16 hours of exposure to UV radiation. It shows the importance of distribution and compatibility of nano particles with the matrix. The methytrimethoxy-silane (MTMS) modified nanoparticles were uniformly distributed in the matrix compared to vinyltriethoxy-silane modified particle [6]. Hence, greater resistance to yellowing and color change in the case of MTMS modified coatings. Figure 2 shows the importance of shape of nano particles. Flake like nano ZnO paricles have high specific surface area (surface area to volume ratio). Hence, resistance to UV degradation was better than spherical nano ZnO particles. Micheal S. Lowry et al. [5] investigated the degree of UV protection afforded by nano ZnO in polyurethane/acrylic topcoat. There was a drop in the UV transmission in nano ZnO modified coating to around 370 nm, which is an indication of UV protecting ability [6]. N.M. Zholobak [7] demonstrated photocatalytic activity of nano ceria. The activity of nano ceria was comparable to that of nano ZnO. In a most recent work, Ahmad Ghasemi Kahrizsang et al. [8] worked on investigating the degradation behavior of modified nano carbon black (CB) epoxy coating by UV irradiation. The result showed that there is a drop in the transmission of UV (B) radiation hence proved that carbon black could also be used as a UV blocker.
Effect on mechanical properties
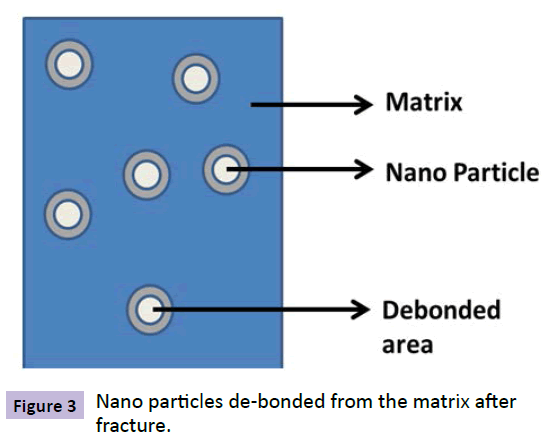
Toughness, yield strength and Mar resistance of the coatings can be improved by using rigid filler materials like inorganic particles. The improvement in the toughness of the coatings can be explained by various mechanisms like crack pinning, crack bridging, micro cracking, crack deflection and shear banding. Only two mechanisms are dominant in the case of nano particles i.e., Plastic void growth and shear banding. Plastic void growth observed by Ma et al. [9] is the main mechanism involved in the toughening of coatings by nano particles. Later Liang and Pearson [10,11] argued that Plastic void growth is not the only mechanism and observed shear yielding near the crack tip region in epoxy coatings with nano silica particles which followed Irwin formalized plastic zone model. The research work carried out by Peerapan Dittanet [12] on nano silica modified epoxy coatings were found to be in good agreement with Liand and pearson argument. Figure 3 shows the de-bonding of nano particles from the matrix after fracture. This de-bonded zone is responsible for plastic zone formation. Work done by many researchers on silica particle effect on epoxy showed good agreement with these theories [13-15].
A recent work by N. Domun [16] on improvement of fracture toughness of epoxy composites using nano-particles gave a comparative illustration of fracture toughness of epoxy composites with respect to different nano particles. The main conclusion drawn was that the nano graphene gave maximum benefit at lower concentrations compared to clay and silica [17]. In a very recent work by Arjun Prakash [18], effect of nano ceria on epoxy was studied and four times improvement in the impact strength was observed. Reinforcement with nano titania showed 57 % improvement in the fractural strength of modified polyester coating with the original polyester. However, the improvement was seen only up to 3 vol % loading. 4 vol% TiO2 reinforcement decreased the toughness value to approximately the value of the original polyester matrix [19].
S. Dhoke and A.S. Khanna [20] improved the mechanical properties of alkyd based water borne coatings by incorporating nano particles with varying concentrations of nano-Al2O3. Mechanical properties including abrasion resistance and scratch resistance were improved significantly. They also incorporated nano ZnO in alkyd based waterborne coatings and used hexa(methoxymethyl)melamine (HMMM) as cross linking agent and found improvements in taber resistance as well as scratch resistance [21,22].
In nano modified Zn epoxy coating, the Young’s modulus was improved by 30 % [23]. In alkyd waterborne coatings, Abrasion resistance was increased with the concentration of Fe2O3. In addition, there was a significant improvement in scratch resistance of the coating. Nano alumina modified epoxy coating has shown improvement in the nano indentation hardness [24]. The adhesion of epoxy adhesive was increased by two times by the addition of 2% nano Al2O3 [25].
Effect on corrosion resistance
Corrosion resistance of the coatings using nano particles can be improved by three ways. First, adding nano particles in the coating matrix which can act as corrosion inhibitors like nano metallic phosphate silicate, titanate or molybdate [26,27]. Second way is to provide barrier properties to the coating. Mica and glass flakes can induce such properties [1]. Third method is providing sacrificial anode method. For example, nano zinc incorporation in the matrix of the primer [23].
S. Dhoke et al. [28] incorporated Fe2O3 and studied the corrosion behavior of water borne alkyd resin as a function of varying concentrations of nano Fe2O3. These nano modified coatings showed the delay in corrosion process even after 30 days of immersion in 3.5% sodium chloride solution. Corrosion resistance of a coating depends on the pigment-binder ratio. This factor can be used to relate the transportation of corrosion species through the coating. The high surface energy of nano fillers result in the low free space between the binder and pigment. This makes the corrosive species difficult to diffuse through the coating, making it resistant to corrosion. But the amount of filler material should be optimum. The amount of resin may not be enough to wet excess nano particles. This may lead to defects. The defect area act like anodes and coated area acts like cathode in corrosive environments. The area of defect is very small that it results in infinite cathode to anode ratio resulting in very high localized corrosion rate [29].
It has been observed that, incorporation of various nano particles like Nano ZnO, Halloysite clay and Al2O3 in the epoxy matrix decreased the corrosion rate of steel substrate by three orders in 0.3% NaCl Solution and two times in 3 wt.% NaCl solution [30]. In the Electrochemical, impedance test it has been found that the coating resistance has been decreased by four orders and coating capacitance has been reduced by three orders implies that the barrier properties are improved and porosity is decreased by nano particle incorporation [30].
Effect on optical properties
The unique property of nano materials is that their wavelength lies in the range of visible light wavelength but the surface energy of these particles is high hence, they tend to agglomerate. This makes it a challenge to incorporate nano fillers without affecting the optical transparency of polymer coating. Thickness of the coating, amount of nano filler and its size, difference in the refractive index of matrix and filler also dictate the optical transparency. Silica nano fillers either in the colloidal or in the fumed form are well known for improving abrasion resistance of coatings. The refractive index is 1.5 for fumed silica [10] hence it would not affect the optical transparency of clear coats whereas alumina is also known to improve the abrasion resistance but its refractive index is 1.7 [20]. Hence, it creates haziness in the appearance. Waterborne alkyd coatings did not show any change in the transmittance and absorbance even after being incorporated with nano ZnO particles. The extent of loss in gloss when exposed to salt spray, humidity and UV radiation was reduced in nano ZnO modified alkyd clear coating [10].
Smart coatings
The coatings, which have ability to sense the changes in environment and respond in a predictable way, are called as smart coatings. These coatings include sensing damage, warning, self-repair and corrosion inhibition. In this review, these coatings are categorized based on their functionality. The following are some important smart coatings.
Hydrophobic coatings
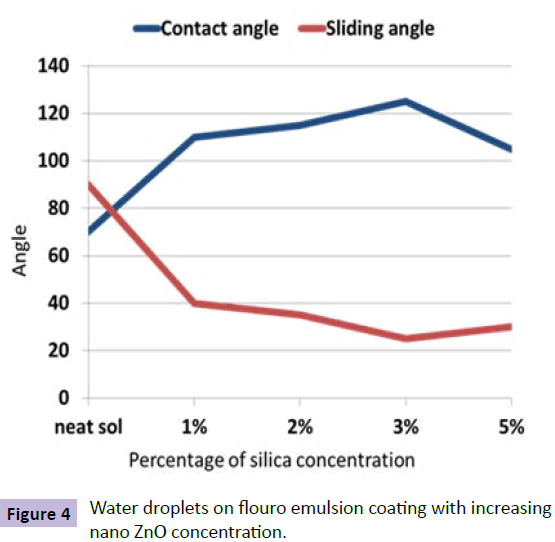
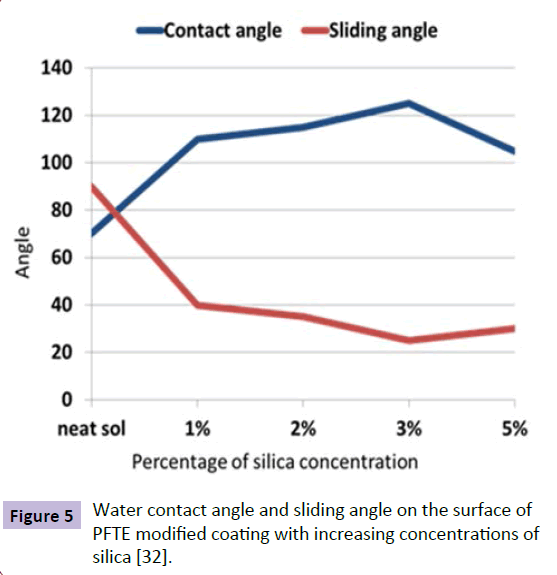
Super hydrophobic coatings are inspired by lotus leaf effect. Lotus leaves have the ability to bead off the water and clean out the contaminations. In order to create such a surface, two pa-rameters are required. Firstly, a very low surface energy and second, nano/micro roughness are required. Surface energy can be reduced by using groups like CF3, CF2, CH3, CH2 etc. Roughness can be induced by various surface treatments like Physical and chemical etching, Plasma treatment, Chemical vapor deposition, Anodic oxidation but these methods are very expensive and complicated. Here nano -particles come into picture. Figure 4 shows water drops on the surface of a hydrophobic coating. Pathak et al. [31] has done similar work on (mlycidyloxypropyltrimethoxysilane) GPTMS, (methyltrimethoxysilane) MTMS and (hexamethoxymethymelamine) HMMM based sol gel coating. He used Flouroalkyl- silane as a Surface tension reducing agent and fumed silica for imparting nano-roughness. With proper optimization of the formulation, they obtained a contact angle of 117o with water. A waterborne silicone polyurethane coating was also developed by them and significant corrosion resistance was observed [32]. Ruchi Grover et al. [33] developed a water repellent organic-inorganic hybrid sol gel coating on aluminium using short chain per-flouro emulsion. They modified nano silica using hexamethyldisilazane and incorporated into the flouro polymer coating for imparting roughness. A contact angle of 125° with sliding angle of 25° was obtained. A roughness of 37nm was obtained when nano ZnO was used as a roughness-imparting agent. Surface energy of HMDZ modified silica is low compared to that of nano ZnO that is why higher levels of loading was possible in the case of fumed silica. So while considering nano particles for imparting surface roughness, Surface energy, Bulk density and working environment must be considered. If the coating requires UV resistance along with hydrophobicity, then nano ZnO or titania should be used. If the coating requires mechanical strength, SiO2 is considered as the best. If corrosion and impact resistance is the concern, nano-CeO2 is an option. Another important factor is optimization of concentration of nano particles in the matrix. Figure 5 shows the importance of optimization of concentration of nano particles. It shows that contact angle decreased after a particular level of concentration of silica in the sol gel coating. Recently, a novel hydrophobic composite was developed by Yi Zhou [34] using photocatalytic TiO2 and perflourotetraethylene mixed with Flouroethylenevinylether. These coatings were multifunctional i.e., both UV resistant and self-cleaning. Water contact angle obtained was 133º. Recently a similar methodology was used to develop a durable self-cleaning coating by Divya Kumar [35] using tetraethylorthosilicate and glicydopropyltriethoxysilane in which flouroalkylsilane and nano silica is used as filler material. A contact angle of 164º and sliding angle of 2º was obtained for these coatings. These coatings showed 96 % efficiency in self-cleaning.
Self-healing coatings
Development of self-healing coatings is inspired from biological systems. The main aim of such development is to incorporate self-healing properties in a coating where the coating can fully or partially recover from mechanical or corrosion damage. Incorporation of urea formaldehyde microcapsules filled with healing polymeric material into the organic coating was the first proposed strategy. N. Sottol [36] had done the first breakthrough in this field in the year 1993. Later a lot of researchers worked in this field and after a lot of successful representations of selfhealing coatings, three approaches became basic for achieving self-healing properties i.e. intrinsic, capsule and vascular methods.
In intrinsic method, material is designed in such a way that reversible bonding takes place after damage. Incorporation of Dicyclopentadiene (DCPD) in the matrix can induce such properties but it is very expensive [37]. Polymers that can undergo thermally assisted reversible reactions can also be used. For example, Modifying aliphatic polyketones to derivatives of furan Furan can undergo Dies- Alder reaction which is a reversible reaction. These modifications were done by Hermosilla [38]. In capsule method, healing material is filled in microcapsules or nano capsules and this healing liquid flows into the ruptured area after damage. This is applicable only for small cracks and only for once. Lot of successful demonstrations has been presented on encapsulation method and this method was extended to multifunctional coatings [36-38]. For example, incorporating corrosion inhibitors in the capsules. These micro or nano containers with healing agents and corrosion inhibitors work in two steps: 1 ) Healing of the damage caused by the corrosion product. 2) Release of inhibitor from the capsule to the damaged area for suppressing corrosion rates. Research over this topic has been done at several levels and incorporating nano containers is a new break through. M.F Montemor [39] successfully represented the efficiency of nano-CeO2 particles as corrosion inhibitors in hybrid silane coatings. Another approach for self-healing of corrosion damage is to design pH sensitive inhibitors. Such design was successfully demonstrated by D Snihirova et al. [40]. They used CaCO3 microbeads saturated with corrosion inhibitors and these beads dissolve when pH becomes 4. This technique is also applicable for nano silica tubes as core with polymer shell [41] and many other inorganic particles like nano TiO2, Hallosite clay and other oxides that can be filled with inhibitors like benzotriazole [42]. The main advantage of the nano capsules is that they can be incorporated in thin coatings but the loading capacity is very low i.e., around 20% [42].
Fire resistant coatings
Fire retardant coatings delay the spreading of fire and provide time for evacuation. Alumino silcates are most commonly used as fire retardants. But the mechanical properties of these coatings are inferior and char is not so stable. To overcome these problems, recently, Silicon based coatings were developed to increase the tolerance temperature to 1000ºC [43]. But these coatings are very expensive. Antimony is another flame retardant but it is toxic in nature. Here, Nano particles come into picture. Nano particles of magnesium and aluminium double layered hydroxides were used as flame retardants. These particles absorb the head and sends out carbon dioxide and water thus reducing overall temperature and forms char upon exposure to fire [44].
Anti-bacterial coatings
Anti-bacterial coatings are those, which suppress the growth of micro-organisms and bacteria. These coatings are especially useful in hospitals and public toilets. Nano copper and silver are proved to be having best inherent anti-bacterial properties. Other metal oxides that can inhibit microbial growth are nano ZnO, CuO, TiO2, MgO [45].
Anti-graffiti coatings
Graffiti is an aesthetic problem and anti-graffiti coatings are one of the best possible solutions. The basic principle is that the surface of the coating will have very low surface tension. Hence resists staining. The main drawback of these coatings is poor mechanical strength. Hence nano SiO2, Al2O3 and nano ZnO are added to improve the scratch resistance and easy to clean property of these coatings [46].
Researchers in Mexico have successfully developed a new type of anti-graffiti paint DELETUM, by functionalizing nano silica particles and polymers to form a coating repellent to water and oil at the same time. As a result, the coated surface is non-stick or very easy to clean, and able to withstand repeated graffiti attack.
Some of the recent on-going work
Since most of the work cited earlier from this lab is for waterborne coatings, the efforts has been made to check whether similar effect of nano particle addition works for solvent based conventional paint systems. In order to check this nano particle addition of ZnO, TiO2 was done for epoxy based coatings (virgin and commercially available lots).
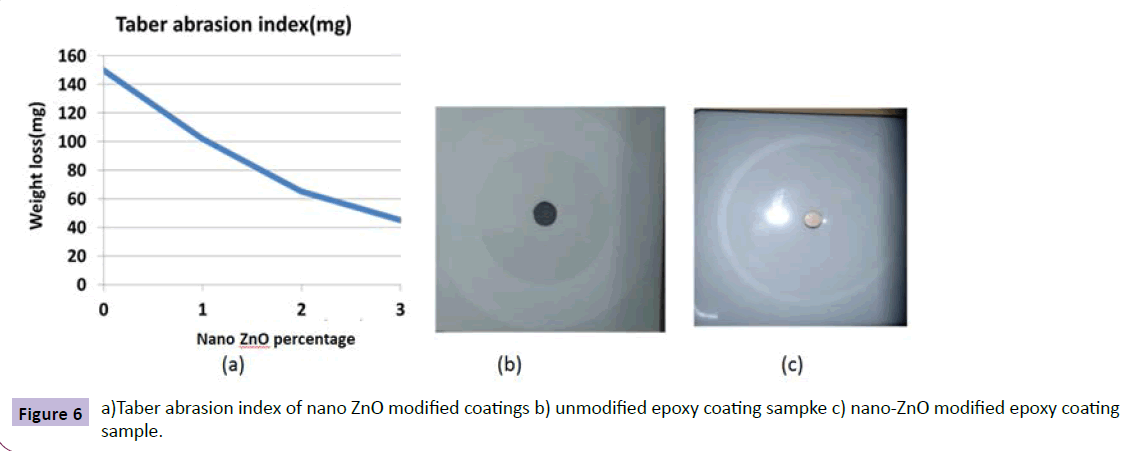
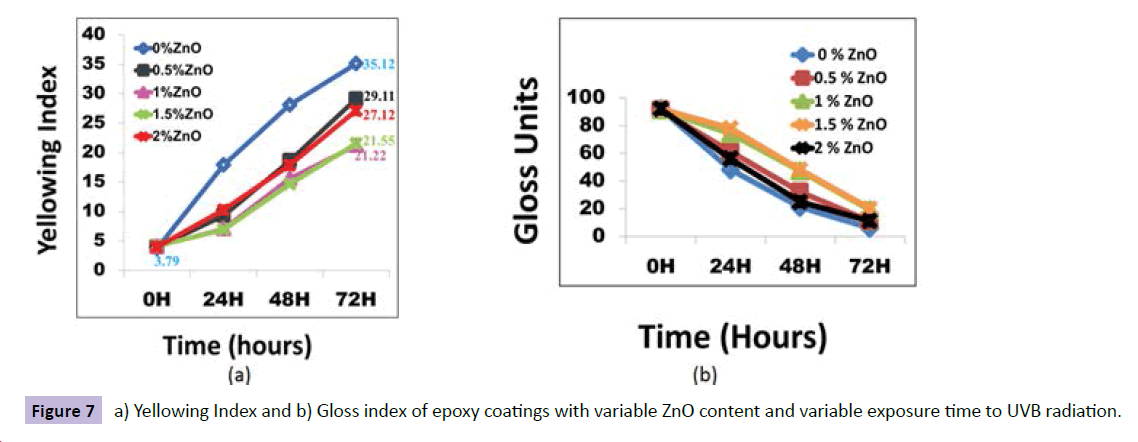
Nano ZnO was incorporated into clear epoxy. And its effect on its mechanical and weathering properties was studied. There was improvement in the abrasion resistance by three times as shown in the Figure 6a from the plot of weight loss vs concentration of nano ZnO in epoxy matrix. Photo-micrographs shown in Figure 6b, 6c, confirms the improvement in the abrasion resistance of nano modified epoxy vs neat one. In the water borne alkyd coatings developed by S.K.Dhoke et al. [21], abrasion resistance of the coatings were improved by 4.5 times with nano ZnO addition. Similar results were obtained when nano-ZnO was incorporated to commercial epoxy. The yellowing index and gloss values obtained after 48 hours of UV exposure is shown in the Figure 7a, 7b for modified and unmodified epoxy coatings respectively. There was 22% improvement in the resistance to yellowing in the case of nano ZnO modified coatings.
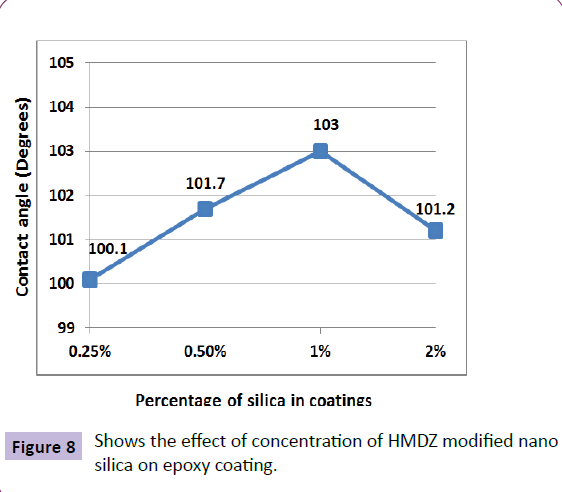
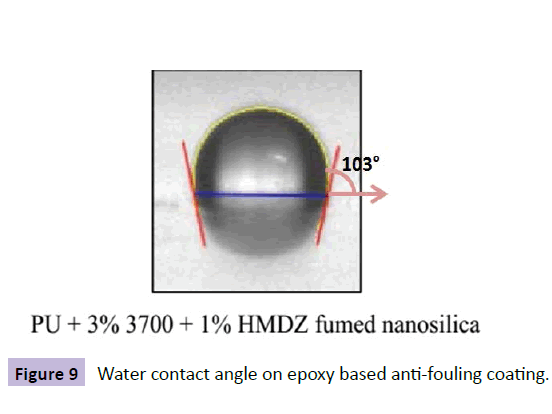
Based upon this success, antifouling coatings were also made in our lab [47]. Various types of silica nano particles were used to develop a low surface energy coating as an alternative to conventional antifouling coatings. Different sizes and surface treated nano silica additives were used in his work. Highest contact angle was achieved in the case of HMDZ modified silica compared to unmodified silica. The importance of concentration of silica is shown in the Figure 8.
Figure 9 shows the water contact angle on the surface of nano silica modified anti-fouling coating.The contact angle obtained in this case was103°. These coatings showed the superior performance behavior and foul release property compared to silicone modified coatings.
Nano particles that can be used for inducing specific property have been tabulated in Table 1.
| Property | Nano material to be used | Reference |
|---|---|---|
| UV resistance | TiO2 (rutile), ZnO | 4 to 6 |
| Mechanical properties | SiO2, ZnO, Al2O3, Zirconia | 7 to 19 |
| Anti -Corrosion | CeO2, TiO2, Fe2O3, Halloysite | 23 to 26 |
| Water repellent | SiO2, ZnO | 29, 31 |
| Self-Cleaning | TiO2, SiO2 | 30, 31 |
| Fire retardant | TiO2, SiO2, Bentonite clay, Halloysite Clay | 40 |
| Anti-Bacterial | Cu, CuO, Ag, ZnO, TiO2 | 41 |
| Anti- Graffiti | SiO2, ZnO, Al2O3 | 42,43 |
Table 1: Different nano particles that can be used for inducing specific property.
Conclusion
Paint industries have been making progress in the development of protective and multifunctional coatings. Application of nano technology has brought a new break-through in this field. This topic has become an interesting subject in the academic research as this can lead to development of innovative industrial coatings. The recent advancements in the use of nano particles in coatings have been reviewed. Formulation of paints for performing a specific function still remains as a technical art. Mechanical properties, Corrosion resistance, UV stability, flame resistance and many properties have been improved significantly at very low concentrations of nano particles incorporation into the coatings thus conserving the natural resources. However there are some draw backs like agglomeration and compatibility of nano particles with the matrix but these can be overcome by methods like silane modification. Another concern is health issues. There is a possibility of release of nano particles into the atmosphere during production of nano particles as well as during modification of paints with nano particles. Some nano-particles like Fe2O3 are proved to be having potential health risks. Hence, strict attention to health aspect is recommended. However, there are more positive aspects of nano particles usage. Exploration of nano-particles can bring out more innovative coatings.
References
- Khanna AS (2008) Nanotechnology in High Performance Paint Coatings. Asian J Exp Sci 21: 25-32.
- Nanoparticles in coatings, European Coatings Journal, 04 (2005) 170.
- Khanna AS High performance organic coatings.
- Yang H et al. (2004) Journal of Applied Polymer Science 92: 3201-3210.
- Rajagopalan N, KhannaAS (2013) International Journal of Scientific and Research Publications 3: 1-14.
- Lowry M (2005) Journal of Coatings Technology and Research5: 233-239
- Zholobak NM, et al. (2011) Journal of Photochemistry and Photobiology B: Biology 102: 32-38.
- Ahmad Ghasemi-Kahrizsangi, Progress in Organic Coatings 85: 199-207.
- Ma J, Mo MS, Du XS, Rosso P, Friedrich K, et al. (2008) Polymer 49: 3510-3523.
- Pearson RA, Yee AF (1986) Materials Science 21: 2462-2474.
- LiangYL, PearsonRA (2009) Polymer 50: 4895-4905.
- Dittanet, Peerapan (2008) The use of nanosilica in epoxy resins. Theses and Dissertations.
- Uddin MF,Sun CT (2009) Effect of Nanoparticle Dispersion on Mechanical Behavior of Polymer Nanocomposites 50th structural Dynamics, and Materials Conference.
- MdSaiful Islam, Hindawi Publishing Corporation Journal of Nanoscience Volume 2013, Article ID 275037.
- Stephan (2013) Sprenger. J Appl Polym Sci 130: 1421–1428.
- Domun N (2015) Nanoscale 7: 1029-1024.
- Sprenger (2015) Nanoscale 7: 1029-1032.
- Arjun Prakash (2015) MIOSR Journal of Mechanical and Civil Engineering (IOSR-JMCE) 3: 2278-1684.
- Evora VMF et al. (2013) Material science and engineering 358-366.
- Dhoke SK, Khanna AS (2009) Materials chemistry and physics 117: 550-556.
- Shailesh K Dhoke (2009) Progress in organic chemistry 64: 39-46.
- Shailesh K Dhoke (2009) Progress in organic chemistry 64: 371-382.
- Xianming Shi,Tuan (2009) Surface & Coatings Technology 204: 237-245.
- KardarP, Ebrahimi M, Bastani S (2008) Progress in Organic Coatings 62: 321-325.
- Zhai L, Ling G (2006) Materials Letters 60: 3031-303.
- Kouloumbi N, Moundoulas P (2002) Pigment and Resin Technol 31: 206-215.
- N. Kouloumbi, et al. (2003)Pigment and resin Technol 32: 89-99.
- Dhoke SK, KhannaAS (2009) JCoat, Tecno, 6: 353-368.
- Shailesh K Dhoke, Khanna AS (2009) Corrosion Science 51: 6-20.
- Xianming Shi (2009) Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating”,Surface& Coatings Technology 204: 237-245.
- Pathak SS, Sharma A,Khanna AS (2009) Progress in Organic Coatings 65: 206-216.
- Pathak SS, Khanna AS (2008) Progress in Organic Coatings62: 409-416.
- Wankhede, Ruchi Grover (2014) Development of hydrophobic inorganic-organic hybrid sol-gel coatings on aluminium using nano-particles, Monash University. Faculty of Engineering. Department of Materials Engineering, Indian Institute of Technology Bombay.
- Yi Zhuo et al. (2015) Ceramics International 41: 5341-5347.
- Divya Kumar (2015) Applied surface science344: 205–212.
- Dry CM, Sottos N (1993) in: K. Varadian (Ed.), Smart Structures and Materials 1995, Smart Materials, Proc. SPIE, 1916: 438–444.
- White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, et al. (2001) Autonomic healing of polymer composites. Lett Nat., 409-794.
- Araya-Hermosilla R, Broekhuis AA, Picchioni F. Reversible polymer networks containing covalent and hydrogen bonding interactions. EurPolym J 50: 127.
- Montemor MF, Pinto R, Ferreira MGS (2009) Electrochim.Acta 54: 5179–5189.
- Snihirova D, Lamaka SV, Montemor MF (2012) Electrochim. Acta 83: 439–447.
- Miccichè F, Fischer H, Varley R, van der Zwaag S (2008) Surf Coat Technol 20: 3346–3353.
- Dmitry G. Shchukin (2013) Polym Chem 4: 4871-4877.
- Takashi Kashiwagi, etal. (2000) Fire Retardancy of Polymer Materials 10: 353-389.
- Yew MC (2015) Progress in organic coatings 78: 59-66.
- LiQ, Mahendra S, Lyon DY, Brunet L, Liga MV,et al. (2008) Water Res 42: 4591-4602
- Swain S, et al. (2013) Transactions on electrical and electronic materials14: 1-8.
- https://www.nanotechproject.org/cpi/products/deletum-5000-anti-graffiti-paint/.
- Lt Cdr Arun, et al. (2014) Development of low surface energy coating as an alternative to conventional anti fouling coatings, Department of Metallurgical Engineering and Material Science, Indian Institute of Technology Bombay.s.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences